Summary
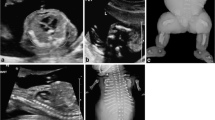
A combined treatment of pregnant mice on day 12 of gestation with both azacytidine and X-irradiation in low doses induces sequence-dependent histological effects. These effects, in turn, induce different symptomatic signs if evaluated either prenatally or neonatally. In the azacytidine treatment/X-irradiation sequence the malformations of the fetal forebrain are predominant. Consequently, these dams show a high incidence in the stillbirth rate. Conversely, the X-irradiation/azacytidine treatment schedule leads only to a mild brain hypoplasia, and does not cause an increased stillbirth rate. In these offspring, however, a severe impairment of small bowel epithelial proliferation capacity was found. This is linked to an outstanding neonatal mortality within 48 h after birth. The pathogenesis of these sequence-dependent effects can be attributed to a selective vulnerability of cells in different stages of the generation cycle. This comprises a high degree of cytolethality affecting the S/G2-stage cells in azacytidine/X-irradiation treatment and the G1/S-stage cells in the reverse combinations (Schmahl 1979). The present observations show the validity of a teratological assay in providing a detailed analysis of the cell kinetic responses after combined noxious influences.
Similar content being viewed by others
References
Bertram JS, Heidelberger C (1974) Cell cycle dependency of oncogenic transformation induced by nitrosoguanidine in culture. Cancer Res 34: 524–537
Dewey WC, Stone LE, Miller HH, Giblak RE (1971) Radiosensitization with 5-bromo-deoxyuridine of Chinese Hamster cells X-irradiated during different phases of the cell cycle. Radiat Res 47: 672–688
Griem ML, Ranninger K (1962) Modification of the radiation effect on hair roots of the mouse by actinomycin. Radiat Res 17:92–100
Kauffman SL (1968) Lengthening of the generation cycle during embryonic differentiation of the mouse neural tube. Exp Cell Res 49: 420–424
Kauffman SL (1975) Kinetics of pulmonary epithelial proliferation during prenatal growth of the mouse lung. Anat Res 183: 393–404
Li LH, Olin R, Fraser TJ, Bhuyan BK (1970) Phase specificity of 5-azacytidine against mammalian cells in tissue culture. Cancer Res 30: 2770–2775
Lloyd HH, Dulmadge EA, Willkoff LJ (1972) Kinetics of the reduction in viability of cultured L 1210 leukemia cells exposed to 5-azacytidine. Cancer Chemother Rep 56: 585–591
Millar JL, Hudspith BN (1976) Sparing effect of cyclophosphamide pretreatment on animals lethally treated with X-irradiation. Cancer Treat Rep 60: 409–414
Miltenberger HG, Korte A (1976) Über die gleichzeitige Wirkung von ionisierender Strahlung und chemischen Agentien auf tierische Zellen. Drug Res 26: 1303–1307
Paterson ARP, Jakobs ES, Lauson GJ, Weinstein WM (1979) Drug sequence-dependent toxicity and small bowel mucosal injury in mice treated with low doses of 3-deazauridine and arabinofuranosylcytosine. Cancer Res 39: 2216–2219
Phelps TA, Blackett NM (1979) Protection of intestinal damage by pretreatment with cytarabine. Int J Radiat Oncol Biol Phys 5: 1617–1620
Phillips TL, Sharan MD, Margolis LW (1975) Modification of radiation injury to normal tissues by chemotherapeutic agents. Cancer 35: 1676–1684
Raake W, Tempel K, Hollatz R (1977) Zur Wirkung von 6-Methyluracil auf Mäuse nach Schädigung durch 2,4,6-Triäthylenimino-1,3,5-triazin oder Ràntgenbestrahlung. Drug Res 27: 132–137
Raedler A, Sievers J (1975) The development of the visual system of the albino rat. Adv Anat Embryol Cell Biol 50:1–88
Schenken LL, Burholt DR, Hagemann RF, Lesher S (1976) The modification of gastrointestinal tolerance and responses to abdominal irradiation by chemotherapeutic agents. Radiology 120: 417–420
Schmahl W (1979) Different teratogenic efficacy to mouse fetal CNS of 5-azacytidine in combination with X-irradiatioin depends on the sequence of successive application. Teratology 19: 63–70
Schmahl W (1982) Kinetics of telecephalic neural cell proliferation during the fetal regeneration period following a single X-irradiation at the late organogenesis stage. I. Sequential study of cell kinetics during the phase of acute events. Radiat Environ Biophys 21: 19–31
Schultze B, Nowak B, Maurer W (1974) Cycle times of the neural epithelial cells of various types of neuron in the rat. An autoradiograpohic study. J Comp Neurol 158: 207–218
Seifertova M, Vesely J, Cihak A (1974) Localization of the labelled 5-azacytidine in cultured mouse embryonic cells. Experientia 30: 1463–1465
Seifertova M, Vesely J, Cihak A, Sorm F (1972) Pycnotic degeneration of ventricular cells in embryonic brain following transplacental exposure to 5-azacytidine. Experientia 28: 841–842
Smith WW, Carter SK, Wilson SM, Newman JW, Cornfield J (1970) Joint lethal effects of actinomycin D and radiation in mice. Cancer Res 30: 51–57
Tolmach LJ, Weiss BG, Hopwood LE (1971) Ionizing radiations and the cell cycle. Fed Proc 30: 1742–1751
Tsubouchi S, Matsuzawa T (1974) Rapid radiation cell death and cell proliferation in intestinal epithelium after 1000 rad irradiation. Radiat Res 57:451–458
UNSCEAR (1982) “Biological effects of radiation in combination with other physical, chemical or biological agents” pg 727–773. Report of the United Nations Scientific Committe on the Effects of Atomic Radiation. Official Record of the General Assembly, 37th Session, Supplement No. 45 (A/37/45). United Nations, New York
Vesely J, Gostof R, Cihak A, Sorm F (1969) Radioprotective effect of 5-azacytidine in AKR mice. Z Naturforsch 24: 318–320
Vesselinovitch SC, Simmons EL, Mihailovich N, Lombard LS, Rav KVN (1972) Additive leukemogenicity of urethan and X-irradiation in infant and young adult mice. Cancer Res 32: 222–225
Withers HR, Elkind MM (1970) Microcolony survival assay for cells of mouse intestinal mucosa exposed to radiation. Int J Radiat Biol 17: 261–267
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schmahl, W. Sequence-dependent toxicity and small bowel mucosal injury in neonatal mice treated with low doses of 5-azacytidine and X-irradiation at the late organogenesis stage. Radiat Environ Biophys 21, 235–245 (1983). https://doi.org/10.1007/BF01341460
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01341460