Summary
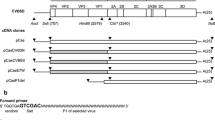
The genetic determinants of plaque size of two variants of coxsackievirus B4, CB4-P and CB4-V, were identified using a panel of recombinant, chimeric viruses. When grown in monkey kidney cells, CB4-V yielded large plaques with an average size of 1.0 cm while CB4-P yielded small plaques with an average size of 0.4 cm. Two genetic domains, the 5′ untranslated region and the VP4 gene sequence, independently influenced plaque size. Recombinant viruses containing the CB4-P genetic background with point mutations in either the VP1 or VP2 coding sequences had small plaque phenotypes. However, two additional chimerics containing the CB4-P genetic background with either a point mutation in the VP4 sequence or four substitutions in the 5′ untranslated region, had large plaque phenotypes. Plaque size correlated with growth kinetics under single-step conditions. Large-plaque variants replicated to higher titers than small-plaque variants. Comparison of the growth kinetics of the recombinant viruses revealed some differences in viral replication. These data suggest that both the 5′ untranslated region and arg-16 of VP4 influence viral replication but at different stages of the replication cycle.
Similar content being viewed by others
References
Bibler-Muckelbauer JK, Kremer M, Tong L, Minor I, Rossmann MG (1994) Towards the structure determination of coxsackie virus B3. Proceedings of the ASV 13th Annual Meeting, Madison, Wisconsin, pp 109
Caggana M, Chan P, Ramsingh A (1993) Identification of a single amino acid residue in the capsid protein VP1 of coxsackievirus B4 that determines the virulent phenotype. J Virol 67: 4797–4803
Hellen C, Wimmer E (1992) Maturation of poliovirus capsid proteins. Virology 187: 391–397
Jenkins O, Booth J, Minor P, Almond J (1987) The complete nucleotide sequence of coxsackievirus B4 and its comparison to other members of the Picornaviridae. J Gen Virol 68: 1835–1848
Kuhn R, Luz N, Beck E (1990) Functional analysis of the internal translation initiation site of foot-and-mouth disease virus. J Virol 64: 4625–4631
McMaster GK, Carmichael GG (1977) Analysis of single and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci USA 74: 4835–4838
Minor PD (1985) Growth, assay and purification of picornaviruses. In: Mahy BWJ (ed) Virology, a practical approach. IRL Press, Oxford, pp 25–41
Moscufo N, Chow M (1992) Myristate-protein interactions in poliovirus: interactions of VP4 threonine 28 contribute to the structural conformation of assembly intermediates and the stability of assembled virions. J Virol 66: 6849–6857
Nakano JH, Hatch MH, Thieme ML, Nottay B (1978) Parameters for differentiating vaccine-derived and wild poliovirus strains. Prog Med Virol 24: 178–206
Nicholson R, Pelletier J, Le S-Y, Sonenberg N (1991) Structural and functional analysis of the ribosome landing pad of poliovirus type 2: in vivo translation studies. J Virol 65: 5886–5894
Pelletier J, Kaplan G, Racaniello V, Sonenberg N (1988) Cap-independent translation of poliovirus mRNA is conferred by sequence elements within the 5′ noncoding region. Mol Cell Biol 8: 1103–1112
Pelletier J, Sonenberg N (1988) Internal initiation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature 334: 320–325
Ramsingh A, Araki H, Bryant S, Hixson A (1992) Identification of candidate sequences that determine virulence in coxsackievirus B4. Virus Res 23: 281–292
Ramsingh A, Hixson A, Duceman B, Slack J (1990) Evidence suggesting that virulence maps to the P1 region of the coxsackievirus B4 genome. J Virol 64: 3078–3081
Ramsingh A, Slack J, Silkworth J, Hixson A (1989) Severity of disease induced by a pancreatropic coxsackie B4 virus correlates with the H-2Kq locus of the major histocompatibility complex. Virus Res 14: 347–358
Rivera VM, Welsh JD, Maizel JV (1988) Comparative sequence analysis of the 5′ noncoding region of the enteroviruses and rhinoviruses. Virology 165: 42–50
Rohll J, Percy N, Ley R, Evans D, Almond J (1994) The 5′-untranslated regions of picornavirus RNAs contain independent functional domains essential for RNA replication and translation. J Virol 68: 4384–4391
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Sanger F, Nicklen S, Coulson AR (1977) Sequencing with chain terminating inhibitors. Proc Natl Acad Sci USA 74: 5463–5467
Skinner MA, Racaniello VR, Dunn G, Cooper J, Minor PD, Almond JW (1989) New model for the secondary structure of the 5′ non-coding RNA of poliovirus is supported by biochemical and genetic data that also show that RNA secondary structure is important in neurovirulence. J Mol Biol 207: 379–392
Toyoda H, Kohara M, Kataoka T, Suganuma T, Omata T, Imura N, Nomoto A (1984) Complete nucleotide sequences of all three poliovirus serotype genomes. Implication for genetic relationship, gene function and antigenic determinants. J Mol Biol 174: 561–585
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ramsingh, A.I., Caggana, M. & Ronstrom, S. Genetic mapping of the determinants of plaque morphology of coxsackievirus B4. Archives of Virology 140, 2215–2226 (1995). https://doi.org/10.1007/BF01323241
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01323241