Summary
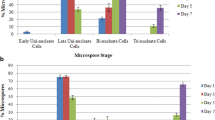
Lucerne (alfalfa —Medicago sativa) pollen, cultured at the late unicellular stage, followed one of two developmental pathways: (1) A pathway involving symmetric mitosis which produces pollen containing two vegetative (2 V) or two generative (2 G) pollen. This morphology was only observed in culture, and pollen which followed this developmental path is defined as non-physiological. Occasionally the formation of multi-nucleate pollen grains containing from 4–9 cells were observed. Sustained divisions were not observed. (2) The production of bicellular (V+G) pollen followed by tricellular (V+2G) pollen. Since these types of grains are encountered during development in vivo, pollen following this developmental pathway is defined as physiological. The proportion of pollen that divided was enhanced by a cold treatment at 4°C for one week, prior to culture. The ratio of non-physiological (i.e., 2V or 2G) to physiological pollen (G+V or 2G+V) was found to be affected by the nature of the osmoticum in the medium. Media containing maltose or melibiose gave higher proportions of non-physiological pollen than media containing glucose or sucrose.
Culture of detached anthers favoured the formation of 2G pollen whereas culture of whole buds favoured the development of 2V pollen. The ratio of non-physiological to physiological pollen after 1 week of culture was used as a criterion for identifying protocols and media which may be more suitable for inducing sustained cell division in lucerne microspores.
Similar content being viewed by others
References
Albertsen MC, Palmer RG (1979) A comparative light and electronmicroscope study of microsporogenesis in male sterile (MS 1) and male fertile soybeans (Glycine max (L.) Merr). Amer J Bot 66: 253–265
Atanossov A, Brown DCW (1984) Plant regeneration from suspension culture and mesophyll protoplast ofMedicago sativa L. Plant Cell Tissue Organ Cult 3: 149–162
Bajaj YPS, Singh H, Gosal SS (1980 a) Haploid embryogenesis in anther cultures of pigeon-pea (Cajanus cajan). Theor Appl Genet 58: 157–159
—, Labana KS, Dhanju MS (1980 b) Induction of pollen-embryos and pollen callus in anther cultures ofArachis hypogaea andA. glabrata. Protoplasma 103: 397–399
—, Ram AK, Labana KS, Singh H (1981) Regeneration of genetically variable plants from the anther-derived callus ofArachis hypogaea andArachis villosa. Plant Sci Lett 23: 35–39
Bhojwani SS, Mullins K, Cohen D (1984) Intra-varietal variation for in vitro plant regeneration in the genusTrifolium. Euphytica 33: 915–921
Blaydes DF (1966) Interaction of kinetin and various inhibitors in the growth of soybean tissue. Physiol Plant 19: 748–753
Breukelen EWM van, Ramanna MS, Hermsen JGTh (1975) Monohaploids (n=x=12) from autotetraploidSolanum tuberosum (2n=4x=48) through two successive cycles of female parthenogenesis. Euphytica 24: 567–574
Brewbaker JL, Kwack BH (1963) The essential role of calcium ion in pollen germination and pollen tube growth. Amer J Bot 50: 859–865
Dale PJ (1975) Pollen dimorphism and anther culture of barley. Planta 127: 213–220
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50: 151–158
Gosal SS, Bajaj YPS (1988) Pollen embryogenesis and chromosomal variation in anther of three food legumes-Cicer arietinum, Pisum sativum andVigna mungo. SABRAO J 20: 51–58
Gharyal PK, Rashid A, Maheshwari SC (1983) Production of haploid plantlets in anther cultures ofAlbizzia lebbeck L. Plant Cell Rep 2: 308–309
Gupta S (1975) Morphogenetic response of haploid callus tissue ofPisum sativun (var. B 22). Indian Agricult 19: 11–21
Heberle-Bors E (1985) In vitro haploid formation from pollen: a critical review. Theor Appel Genet 71: 361–374
Hewitt EJ (1966) Sand and water culture methods used in the study of plant nutrition. Technical communication no. 22, 2nd edn. Commonwealth Bureau of Horticulture and Plantation Crops, Eastern Press, London
Horner M, Street HE (1978) Pollen dimorphism — origin and significance in pollen plant formation by anther culture. Ann Bot 42: 763–771
Hu H, Zeng JZ (1984) Development of new varieties via anther culture. In: Ammirato PV et al (eds) Handbook of plant cell culture, vol 3. Macmillan, New York, pp 64–90
McCoy TJ, Bingham ET (1988) Cytology and cytogenetics of alfalfa. In: Hanson AA, Barnes DK (eds) Alfalfa and alfalfa improvement. Agronomy monograph no. 29. ASA-CSSA-SSSA, Madison, pp 737–776
—, Smith LY (1983) Genetics, cytology, and crossing behaviour of an alfalfa (Medicago sativa) mutant resulting in failure of the postmeiotic cytokinesis. Can J Genet Cytol 25: 390–397
Mokhtarzadeh A, Constantin MJ (1978) Plant regeneration from hypocotyl and anther derived callus of Berseem clover. Crop Sci 18: 567–572
Murishige T, Skoog F (1962) A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiol Plant 15: 473–497
Niizeki M, Grant WF (1971) Callus, plantlet formation, and polyploidy from cultured anthers ofLotus andNicotiana. Can J Bot 49: 2041–2051
Peters JE, Crocomo OJ, Sharp WR, Paddock EF, Tegenkamp I, Tegenkamp T (1977) Haploid callus cells from anthers ofPhaseolus vulgaris. Phytomorphology 27: 79–85
Phillips GC, Collins GB (1979) In vitro tissue culture of selected legumes and plant regeneration from callus cultures of red clover. Crop Sci 19: 59–64
Rao PVL, De DN (1987) Haploid plants from in vitro anther culture of the leguminous tree,Peltophorum pterocarpum (DC) K. Hayne (Copper pod). Plant Cell Tissue Organ Cult 11: 167–177
Sator C (1985) Regeneration von Lupinenpflanzen aus Antheren. Landbauforschung Volkenrode 35: 5–7
Saunders JW, Bingham ET (1972) Production of alfalfa plants from callus tissue. Crop Sci 12: 804–808
Schenck RU, Hildebrandt AC (1972) Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can J Bot 50: 199–204
Stuart DA, Strickland SG (1984) Somatic embryogenesis from cell cultures ofMedicago sativa L. 2. The interaction of amino acids with ammonium. Plant Sci Lett 34: 175–181
Sunderland N (1982) Induction of growth in the culture of pollen. Symp Br Soc Cell Biol 4: 1–24
—, Dunwell JM (1977) Anther and pollen culture. In: Street HE (ed) Plant tissue and cell culture, 2nd edn. Blackwell, Oxford, pp 223–265
Tomes DT, and Peterson RL (1981) Isolation of a dwarf plant responsive to exogenous GA 3 from anther cultures of birdsfoot trefoil. Can J Bot 59: 1338–1342
Uchimiya H, Murshige T (1974) Evaluation of parameters in the isolation of protoplasts from cultured tobacco cells. Plant Physiol 54: 936–944
Xu S (1979) Successful induction of alfalfa pollen haploid plant. Plant Mag 6: 5
Zagorska N, Robeva P, Dimetrov V, Shtreva R, Gancheva V (1984) Induction of regeneration in anther cultures inMedicago sativa L. Comp Rend Acad Bulgare Sci 37: 1099–1102
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tanner, G.J., Piccirilli, M., Moore, A.E. et al. Initiation of non-physiological division and manipulation of developmental pathway in cultured microspores ofMedicago sp.. Protoplasma 158, 165–175 (1990). https://doi.org/10.1007/BF01323129
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01323129