Summary
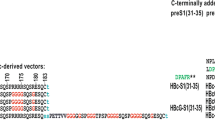
The amino acid sequence encoded by the preS1 region of hepatitis B virus genome is expressed on the surface of virions and subviral particles. The preS1 region is involved in the recognition of specific receptors responsible for the attachment of HBV to the host cell. The cell receptor binding site was assigned to the preS1 (20–47 aa) fragment. In order to obtain a large quantity of preS1 binding domains of HBV the expression vector pWX4 was constructed. It contains four tandemly joined DNA sequences, each coding for preS1 (20–49 aa), fused with the 3′ end of a DNA fragment coding for 450 aa of β-galactosidase.E. coli cells transformed with this vector produce fusion protein β-gal-preSlx4 in the form of inclusion bodies. Owing to the specific trypsin digestion, the preSlx4 domain was cleaved from the fusion protein. The resulting product, a 16 kDa protein, was isolated and purified by anion exchange chromatography. The presence of four Asp-Pro bonds in this sequence and the primary structure of the first 28 N-terminal amino acids were determined. Following the confirmation of the antigenic properties, the recombinant preS1 protein was used for detection of the anti-preS1 response in sera from HBV infected patients.
Similar content being viewed by others
References
Alberti A, Gerlich WH, Heermann K-H, Pontisso P (1990) Nature and display of hepatitis B virus envelope proteins and the humoral immune response. Semin Immunopathol: 12: 5–23
Birnboim HC, Doly J (1979) A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res 7: 1513–1523
Budkowska A, Dubreuil P, Capel F, Pillot J (1986) Hepatitis B virus preS gene encoded antigenic specificity and anti-preS1 antibody: relationship between anti-preS response and recovery. Hepatology 6: 360–368
Budkowska A, Dubreuil P, Mailard P, Polnard T, Pillot J (1990) A biphasic pattern of anti-preS-responses in acute hepatitis B virus infection. Hepatology 12: 1271–1277
Budkowska A, Dubreuil P, Poynard T, Marcellin P, Loriot M-A, Maillard P, Pillot J (1992) Anti-preS responses and viral clearance in chronic hepatitis B virus infection. Hepatology 1: 26–31
Canfield RE, Anfinsen ChB (1963) Primary structure of specific proteins: composition, structure and function. In: Neurath H (ed) The proteins. Academic Press, New York London, pp 357–371
Chung CT, Miller RH (1988) A rapid and convenient method for the preparation and storage of competent bacterial cells. Nucleic Acids Res 16: 3580–3580
Coursaget P, Buisson Y, Bourdil C, Yvonnet B, Moline C, Diop MT, Chiron JP, Bao O, Diop-Mar J (1990) Antibody response to preS1 in hepatitis B virus induced liver disease and after immunization. Res Virol 141: 536–570
Gerlich W (1991) Hepatitis B surface proteins. J Hepatol 13: 90–92
Gerlich WH, Heermann K-H, Lu X (1992) Functions of hepatitis B surface proteins. Arch Virol [Suppl] 4: 129–132
Guo LH, Stepien PP, Tso JY, Brousseau R, Narang S, Thomas DY, Wu R (1984) Synthesis of human insulin gene, VIII. Construction of expression vectors for fused proinsulin production inEscherichia coli. Gene 29: 251–254
Hanahan D (1983) Studies on transformation ofEscherichia coli with plasmids. J Mol Biol 166: 567–580
Heermann KH, Goldmann U, Schwartz W, Seyffarth T, Baumgarten H, Gerlich WH (1984) Large surface proteins of hepatitis B virus containing the preS sequence. J Virol 52: 396–402
Itakura K, Hirose T, Crea R, Riggs AD, Heyneker HL, Boyer HW (1977) Expression inEscherichia coli of a chemically synthesized gene for the hormone somatostatin. Science 198: 1056–1063
Kadakura H, Yoda K, Imai M, Yamasaki M (1990) Production and secretion in Escherichia coli of hepatitis B virus pre-S2 A antigen as fusion proteins with β-lactamase. Appl Environ Microbiol 9: 2742–2747
Klinkert MQ, Theilmann L, Pfaff E, Schaller H (1986) PreS1 antigens and antibodies early in the course of acute hepatitis B virus infection. J Virol 58: 522–526
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685
Lin Y, Liu YX, Cislo T, Mason BL, Yu M-YW (1991) Expression and characterization of the PreS1 peptide of hepatitis B surface antigen inEscherichia coli. J Med Virol 33: 181–187
Maniatis T, Fritsch EF, Sambrook J (1992) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Marvin HJP, Withold B (1987) A highly efficient procedure for quantitative formation of intact and viable lysozyme sferoplasts fromE. coli. Anal Bichem 164: 320–331
Maxam AM, Gilbert W (1979) Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol 65: 499–560
Messing J (1979) A Multipurpose cloning system based on the single-stranded DNA bacteriophage M13. Recombinant DNA technical bulletin, NIH publication 79–99, 2: 43–48
Nakane P, Kawai A (1974) Peroxidase labeled antibody. A new method of conjugation. J Histochem Cytochem 22: 1084–1091
Neurath AR, Kent SBH, Strick N (1986) Identification and chemical synthesis of a host receptor binding site on hepatitis-B virus. Cell 46: 429–436
Neurath AR, Seto B, Strick N (1989) Antibodies to synthetic peptides from the anti-pres-S1 region of the hepatitis B virus (HBV) envelope (env) protein are virus-neutralizing and protective. Vaccine 7: 234–236
Neurath AR, Strick N, Sproul P, Ralph HE, Valinsky J (1990) Detection of receptors for hepatitis B virus on cells of extrahepatic origin. Virology 176: 448–457
Pontisso P, Ruvoletto MG, Gerlich WH, Heermann KH, Bardini R, Alberti A (1989) Identification of an attachment site for human liver plasma membranes B virus particles. Virology 173: 522–530
Sułowska Z, Tchórzewski H, Sidorkiewicz M, Płucienniczak A (1900) The effect of preS1 protein in longterm cultures of human lymphocytes after hepatitis B immunization. Arch Immunol Ther Exp 40: 275–281
Sułowska Z, Dworniak D, Tchorzewski H, Zeman K, Sidorkiewicz M (1995) Effect of preS1 antigen on human lymphocyte proliferative responses. Immunol Lett (in press)
Theilmann L, Burkhardt H-D, Galle PR, Gmelin K, Kommerell B, Pfaff E (1988) Detection of antibodies against preS1 proteins in sera of patients with hepatitis B virus infection by Elisa using a perS fusion protein expressed inE. coli. Alzheimer Forsch Drug Res 38: 1856–1858
Towbin H, Stachelin TH, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets procedures and some applications. Proc Natl Acad Sci USA 76: 4350–4354
Uznanski B, Grajkowski A, Wilk A (1989) The convenient base protecting isopropoxyacetyl group of solid-support synthesis of oligodeoxyribonucleotides and their triester analogues. Nucleic Acids Res 17: 4863–4877
Van Huynh N, De Bacher O, Decleire M, Colson C (1989) A procedure for the preparation of bacterial DNA that employs dimethyl sulfoxide to induce the lysis of cells. Anal Biochem 176: 464–467
Yanisch-Perronn C, Vieira J, Messing J (1985) Improved MB phage cloning vestors and host strains: nucleotide sequences of MB mp18 and pUC19 vectors. Gene 33: 109–119
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sidorkiewicz, M., Płucienniczak, G. & Płucienniczak, A. Expression and characterization of the multiplied, recombinant preS1 antigen of hepatitis B virus. Archives of Virology 140, 1935–1944 (1995). https://doi.org/10.1007/BF01322683
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01322683