Summary
This paper describes the role of actin filaments in setting up the phragmosome — the transvacuolar device that anticipates the division plane — and in forming a supracellular system that seems to override cell boundaries.
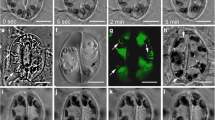
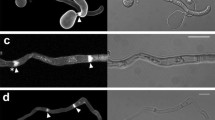
Tradescantia leaf epidermal cells were induced to divide by wounding the leaf. New division planes formed parallel to slits, and encircled puncture wounds — the new division planes lining up across cells, instead of the joints being off-set as in normal, unwounded tissue. Within 30 min after wounding, rhodamine phalloidin staining showed that a belt of fine, cortical actin filaments formed parallel to the wound. In the next stage, migration of nuclei to a wall adjacent to the wound, involved pronounced association of actin filaments with the nucleus. Migration could be inhibited with cytochalasin D, confirming the role of actin in traumatotaxis. Later still, actin strands were seen to line up from cell to cell, parallel to the wound, anticipating the future division plane. Next, actin filaments accumulated in this anticlinal plane, throughout the depth of the cell, thereby contributing to the formation of the phragmosome. The phragmosome has been shown in previous work (Flanders et al. 1990) to contain microtubules that bridge nucleus to cortex, and is now found to contain actin filaments. Actin filaments are therefore involved in the key stages of nuclear migration and division plane alignment. The supracellular basis of actin alignment is discussed.
Similar content being viewed by others
References
Bakhuizen R, Van Spronsen PC, Sluiman-Den Hertog FAJ, Venverloo CJ, Goosen-de-Roo L (1985) Nuclear envelope radiating microtubules in plant cells during interphase mitosis transition. Protoplasma 123: 43–51
Biggs AR (1985) Detection of impervious tissue in tree bark with selective histochemistry and fluorescence microscopy. Stain Technol 60: 299–304
Bünning E (1959) Die Wirkung von Wundreizen. Traumatotropismus, Traumatonastie, Traumatotaxis, Traumatodinese, Traumatochorismus. In: Bünning E (ed) Physiologie der Bewegungen, Teil 1, Bewegungen durch Einflüsse mechanischer und elektrischer Natur sowie Strahlungen. Springer, Berlin Göttingen Heidelberg, pp 119–134 [Ruhland W (ed) Handbuch der Pflanzenphysiologie—Encyclopedia of Plant Physiology, vol 17]
Flanders DJ, Rawlins DJ, Shaw PJ, Lloyd CW (1990) Nucleus-associated microtubules help determine the division plane of plant epidermal cells: avoidance of 4-way junctions and the role of cell geometry. J Cell Biol 110: 1111–1122
Iwata K, Hogetsu T (1989) The effects of light irradiation on the orientation of microtubules in seedlings ofAvena sativa L andPisum sativum L. Plant Cell Physiol 30: 1011–1016.
Kirschner H, Sachs T (1978) Cytoplasmic reorientation: an early stage of vascular differentiation. Israel J Bot 27: 131–137
Korn RW (1980) The changing shape of plant cells: transformations during cell proliferation. Ann Bot 46: 649–666
Lintilhac PM, Vesecky TB (1981) Mechanical stress and cell wall orientation in plants. II. The application of controlled directional stress to growing plants: with a discussion on the nature of the wound reaction. Amer J Bot 68: 1222–1230
Lloyd CW, Pearce KJ, Rawlins DJ, Ridge RW, Shaw PJ (1987) Endoplasmic microtubules connect the advancing nucleus to the tip of legume root hairs, but F-actin is involved in basipetal migration. Cell Motil Cytoskeleton 8: 27–36
—, Traas JA (1988) The role of F-actin in determining the division plane of carrot suspension cells. Drug studies. Development 102: 211–222
Miehe H (1901) Über Wanderungen des pflanzlichen Zellkerns. Flora 88: 105–142
Pickett-Heaps JD, Northcote DH (1966) Organization of microtubules and endoplasmic reticulum during mitosis and cytokinesis in wheat meristems. J Cell Sci 1: 109–120
Roberts IN, Lloyd CW, Roberts K (1985) Ethylene-induced microtubule re-orientation: mediation by helical arrays. Planta 164: 439–447
Sachs T (1972) The pattern of plasmolysis as a criterion for intercellular relations. Israel J Bot 21: 90–98
Schnepf E, von Traitteur R (1973) On the traumatotactic movement of nuclei inTradescantia leaves. Z Pflanzenphysiol 69: 181–184
Sinnott EW, Bloch R (1940) Cytoplasmic behaviour during division of vacuolate plant cells. Proc Natl Acad Sci USA 26: 223–227
— — (1941 a) The relative position of cell walls in developing plant tissues. Amer J Bot 28: 607–617
— — (1941 b) Division in vacuolate plant cells. Amer J Bot 28: 225–232
— — (1945) The Cytoplasmic basis of intercellular patterns in vascular differentiation. Amer J Bot 32: 151–156
Sonobe S, Shibaoka H (1989) Cortical fine actin filaments in higher plant cells visualized by rhodamine-phalloidin after pre-treatment with m-maleimidobenzoyl N-hydroxysuccinimide ester. Protoplasma 148: 80–86
Traas JA, Doonan JH, Rawlins DJ, Shaw PJ, Watts J, Lloyd CW (1987) An actin network is present in the cytoplasm throughout the cell cycle of carrot cells and associates with the dividing nucleus. J Cell Biol 105: 387–395
Venverloo CJ, Hovenkamp PH, Weeda AJ, Libbenga KR (1980) Cell division inNautilocalyx explants. I. Phragmosome, preprophase band and plane of division. Z Pflanzenphysiol 100: 161–174
—, Libbenga KR (1987) Regulation of the plane of cell division in vacuolated cells. I. The function of nuclear positioning and phragmosome formation. J Plant Physiol 131: 267–284
Wilms FHA, Derksen J (1988) Reorganization of cortical microtubules during cell differentiation in tobacco explants. Protoplasma 146: 127–132
Author information
Authors and Affiliations
Additional information
Dedicated to the memory of Professor Oswald Kiermayer
Rights and permissions
About this article
Cite this article
Goodbody, K.C., Lloyd, C.W. Actin filaments line up acrossTradescantia epidermal cells, anticipating wound-induced divison planes. Protoplasma 157, 92–101 (1990). https://doi.org/10.1007/BF01322641
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01322641