Summary
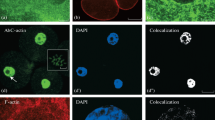
Changes in mammary gland tubulin were studied immunocytochemically during transition from late pregnancy to lactation. Indirect immunofluorescence was used to localize tubulin in mammary glands from late pregnant, early lactating and peak lactating guinea pigs. Whole rabbit antiserum against guinea pig brain tubulin and affinity-purified antibody indicated increases in alveolar cell tubulin content from late pregnancy through peak lactation coincident with the development of lactation. Only alveolar cells displayed high, specific fluorescence or underwent a developmental increase. Tubulin was concentrated apically, in association with secretory structures. In a second study comparing mammary tissues from 18 days pregnant and 10 days lactating rats, EM immunogold was used with three commercial antitubulins ranging from a rabbit polyclonal antiserum against chick brain MTs to a monoclonal mouse anti-alpha tubulin. Gold particle counts indicated 2- to 5- fold tubulin increases in alveolar cells with lactation and development of an apicobasal (high apical) tubulin gradient. Variations among the three anti-tubulins is discussed. The results confirmed previous observations of whole gland tubulin increases based on colchicine binding assays and localized the site of the increase primarily in alveolar cells.
Similar content being viewed by others
Abbreviations
- EM:
-
electron microscope-(ic)
- GAM:
-
goat anti-mouse
- GAR:
-
goat anti-rabbit
- Ig:
-
immunoglobulin
- MC:
-
monoclonal
- MT:
-
microtubule
- PAGE:
-
polyacrylamide gel electrophoresis
- PIPES:
-
1,4-piperazine diethane sulfonic acid
- PBS:
-
phosphate buffered saline
- PC:
-
polyclonal
- Rb:
-
rabbit
- SDS:
-
sodium dodecyl sulfate
References
Armbruster BL, Wunderli H, Turner BM, Raska I, Kellenberger E (1983) Immunocytochemical localization of cytoskeletal proteins and Histone 2 B in isolated membrane-depleted nuclei, metaphase chromatin, and whole Chinese hamster ovary cells. J Histochem Cytochem 31: 1385–1393
Brinkley BR, Fuller GM, Highfield DP (1975) Cytoplasmic microtubules in normal and transformed cells in culture: analysis by tubulin antibody immunofluorescence. Proc Natl Acad Sci USA 72: 4981–4985
De Mey J, Moeremans M, Geuens G, Nuydens R, De Brabander M (1981) High resolution light and electron microscopic localization of tubulin with the IGS (Immuno Gold Staining) method. Cell Biol Int Rep 5: 889–899
Dustin P, Flament-Durand J, Couck AM, Depierreux M (1980) Vincrinstine-induced tubulin paracrystals in the nuclei of mammalian neurons. J Submicrosc Cytol 12: 611–616
Dylewski D, Keenan TW (1983) Compound exocytosis of casein micelles in mammary epithelial cells. Eur J Cell Biol 31: 114–124
Franke WW, Luder MR, Kartenbeck J, Zerban H, Keenan TW (1976) Involvement of vesicle coat material in casein secretion and surface regeneration. J Cell Biol 69: 173–195
Fuller GM, Brinkley BR, Boughter JM (1975) Immunofluorescence of mitotic spindles by using monospecific antibody against bovine brain tubulin. Science 187: 948–950
Guerin MA, Loizzi RF (1978) Inhibition of mammary gland lactose secretion by colchicine and vincristine. Am J Physiol (Cell Physiol) 3: 234-C177–180
— — (1980) Tubulin content and assembly states in guinea pig mammary gland during pregnancy and lactation. Proc Soc Exp Biol Med 165: 50–54
Gunderson GG, Bulinski JC (1986) Microtubule arrays in differentiated cells contain elevated levels of a post-translationally modified form of tubulin. Eur J Cell Biol 42: 288–294
Houdebine LM, Ollivier-Bousquet M, Djiane J (1982) Role des proteines membranaires liant la colchicine dans la transmission du message prolactinique aux genes des caseines dans la glande mammaire de lapine. Biochemie 64: 21–28
Houliston E, Maro B (1989) Posttranslational modification of distinct microtubule subpopulations during cell polarization and differentiation in the mouse preimplantation embryo. J Cell Biol 108: 543–551
Knudson CM, Stemberger BH, Patton S (1978) Effects of colchicine on ultrastructure of the lactating mammary cell: membrane involvement and stress on the Golgi apparatus. Cell Tissue Res 195: 169–181
Kuhn NR (1969) Progesterone withdrawal as the lactogenic trigger in the rat. J Endocrinol 44: 39–54
Kurihara H, Uchida K (1987) Distribution of microtubules and microfilaments in exocrine (ventral prostatic epithelial cells and pancreatic exocrine cells) and endocrine cells (cells of the adenohypophysis and islets of Langerhans). The relationship between cytoskeletons and epithelial cell polarity. Histochemistry 87: 223–227
Lacy PE, Malaisse WJ (1973) Microtubules and beta cell secretion. Recent Progr Horm Res 29: 198–228
—, Howell SL, Young DA, Fink CJ (1968) New hypothesis of insulin secretion. Nature 219: 1177–1179
Loizzi RF (1983) Ovariectomy-induced tubulin polymerization in pregnant rat mammary gland. Proc Soc Exp Biol Med 173: 252–255
— (1985) Progesterone withdrawal stimulates mammary gland tubulin polymerization in pregnant rats. Endocrinology 116: 2543–2547
—, Guerin MA (1979) Multiple biopsies of guinea pig mammary glands during pregnancy and lactation. Lab Animal Sci 29: 221–223
—, dePont JJHH, Bonting SL (1975) Inhibition by cyclic AMP of lactose production in lactating guinea pig mammary gland slices. Biochim Biophys Acta 392: 20–25
Menko AS, Tan KB (1980) Nuclear tubulin of tissue culture cells. Biochim Biophys Acta 629: 359–370
Nickerson SC, Keenan TW (1979) Distribution and orientation of microtubules in milk secreting epithelial cells of rat mammary gland. Cell Tissue Res 202: 303–312
—, Smith JJ, Keenan TW (1980 a) Role of microtubules in milk secretion — Action of colchicine on microtubules and exocytosis of secretory vesicles in rat mammary epithelial cells. Cell Tissue Res 207: 361–376
— — — (1980 b) Ultrastructural and biochemical response of rat mammary epithelial cells to vinblastine sulfate. Eur J Cell Biol 23: 115–121
Ollivier-Bousquet M, Denamur R (1973) Inhibition par la colchicine de la secretion des proteines du lait. C R Acad Sci (IV) 276: 2183–2186
Patton S (1974) Reversible suppression of lactation by colchicine. FEBS Lett 48: 85–87
— (1976) Mechanisms of secretion: effects of colchicine and vincristine on composition and flow of milk in the goat. J Dairy Sci 59: 1414–1419
—, Stemberger BH, Knudson CM (1977) The suppression of milk fat globule secretion by colchicine: an effect coupled to inhibition of exocytosis. Biochim Biophys Acta 499: 404–410
Pencek PF, Loizzi RF (1981) Morphological characterization and tubulin status of several populations of alveolar cells isolated from lactating guinea pig mammary gland. J Cell Biol 91: 325 a
Piperno G, Le Dizet M, Chang X (1987) Microtubules containing alpha-tubulin in mammalian cells in culture. J Cell Biol 104: 289–302
Pitelka DR, Kerkof PR, Gange HT, Smith S, Abraham S (1969) Characteristics of cells dissociated from mouse mammary gland. Exp Cell Res 57: 43–62
Ravindra R, Grosvenor CE (1988) Soluble and polymerized tubulin levels in the anterior pituitary lobe of the lactating rat during suckling. Endocrinology 122: 114–119
Shelanski M, Gaskin F, Canter C (1973) Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci USA 70: 765–768
Sherline P, Schiavone K (1977) Immunofluorescence localization of proteins of high molecular weight along intracellular microtubules. Science 198: 1038–1040
—, Lee YC, Jacobs LS (1977) Binding of microtubules to pituitary secretory granules and secretory membranes. J Cell Biol 72: 380–389
Virtanen I (1986) Microtubule disruption does not prevent intracellular transport and secretory processes of cultured fibroblasts. Eur J Cell Biol 42: 281–287
Weber K, Rathke PC, Osborn M (1978) Cytoplasmic microtubular images in glutaraldehyde-fixed tissue culture cells by electron microscopy and by immunofluorescence microscopy. Proc Natl Acad Sci USA 75: 1820–1824
Weisenberg R (1972) Microtubule formation in vitro in solutions containing low calcium concentrations. Science 177: 1104–1105
Zisapel N, Levi M, Gozes I (1980) Tubulin: an integral protein of mammalian synaptic vesicle membranes. J Neurochem 34: 26–32
Author information
Authors and Affiliations
Additional information
Dedicated to Professor Stuart Patton on the occasion of his 70th birthday.
Rights and permissions
About this article
Cite this article
Loizzi, R.F., Shao, D. Tubulin increases with lactation in rat and guinea pig mammary alveolar cells. Protoplasma 159, 129–143 (1990). https://doi.org/10.1007/BF01322596
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01322596