Summary
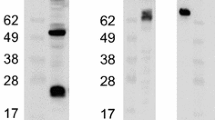
The intracellular processing and antigenic maturation of the measles virus (MV) hemagglutinin (H) protein in virus infected cells were probed with murine monoclonal antibodies (Mabs) that reacted with continuous and discontinuous epitopes. The antibodies distinguished between the immature, cotranslational monomeric form of the protein and the mature, dimeric hemagglutinin structure. This was evidenced by testing of immunoreactivity of the Mabs with synthetic peptides, by in vitro synthesized H protein analysis, and by pulse-chase analysis of gel separated monomeric and dimeric forms of the H protein. Time kinetics analysis showed that the protein was synthesized as monomers and most of them were converted into dimers witht 1/2 about 30 min. The H protein remained endoglycosidase H (Endo H) sensitive up to 30 min and started to acquire partial resistance to Endo H between 30 and 60 min (t 1/2 about 60 min) after synthesis. Oligomerization of the H protein was unaffected in virus infected cells treated with a compound (carbonylcyanide m-chlorophenylhydrazone, CCCP) that blocks transport from the endoplasmic reticulum (ER) to the Golgi complex. These results suggest that the H protein dimerization takes place in the ER before its transport to the medial Golgi complex. The Mabs specific for discontinuous epitopes reacted with the H protein in cells treated with CCCP. Thus conformational antigenic epitope formation appears to take place in the ER.
Similar content being viewed by others
References
Åkerlind-Stopner B, Utter G, Mufson MA, Örell C, Lerner RA, Norrby E (1990) A subgroup-specific antigenic site in the G protein of respiratory syncytial virus forms a disulfide-bonded loop. J Virol 64: 5143–5148
Alkhatib G, Briedis DJ (1986) The predicted primary structure of the measles virus hemagglutinin. Virology 150: 479–490
Boulay F, Doms RW, Webster RG, Helenius A (1988) Posttranslational oligomerization and cooperative acid activation of mixed influenza hemagglutinin trimers. J Cell Biol 106: 629–639
Carter MJ, Willcocks MM, Löffer S, ter Meulen V (1982) Relationship between monoclonal antibody biding sites on the measles virus haemagglutinin. J Gen Virol 63: 113–120
Copeland CS, Doms RW, Bolzau EM, Webster RG, Helenius A (1986) Assembly of influenza hemagglutinin trimers and its role in intracellular transport. J Cell Biol 103: 1179–1191
Copeland CS, Zimmer KP, Wagner KR, Healey GA, Mellman I, Helenius A (1988) Folding, trimerization, and transport are sequential events in the biogenesis of influenza virus hemagglutinin. Cell 53: 197–209
Doms RW, Keller DS, Helenius A, Balch WE (1987) Role of triphosphate in regulating the assembly and transport of vesicular stomatitits virus G protein trimers. J Cell Biol 105: 1957–1969
Doms RW, Ruusala A, Machamer CM, Helenius A, Rose JK (1988) Differential effects of mutations in three domains on folding, quaternary structure, and intracellular transport of vesicular stomatitis virus G protein. J Cell Biol 107: 89–99
Doms RW, Lamb RA, Rose JK, Helenius A (1993) Minireview: folding and assembly of viral membrane proteins. Virology 193: 545–562
Eschle D (1988) Diploma thesis. University of Zurich, Zurich, Switzerland
Freeman RB (1984) Native disulphide bond formation in protein biosynthesis: evidence for the role of protein disulphide isomerase. Trends Biochem Sci 9: 438–441
Fries E, Rothman JE (1980) Transport of vesicular stomatitis virus glycoprotein in a cell-free extract. Proc Natl Acad Sci USA 77: 3870–3874
Gething MJ, Mclammon K, Sambrook J (1986) Expression of wild type and mutant forms of influenza hemagglutinin: the role of folding in intracellular transport. Cell 46: 939–950
Gombart AF, Hirano A, Wong TC (1993) Conformational maturation of measles virus nucleocapsid protein. J Virol 67: 4133–4141
Houghten RA (1985) General method for the rapid solid phase synthesis of large numbers of peptides: specificity of antigenantibody interaction at the level of individual amino acids. Proc Natl Acad Sci USA 82: 5131–5135
Houghten RA, Bray MK, DeGraw ST, Kirby CJ (1986) Simplified procedure for carrying out simultaneous multiple hydrogen fluoride cleavage of protected peptide resins. Int J Peptide Protein Res 27: 675–680
Hu A, Sheshberadaran H, Norrby E, Kövamees J (1993) Molecular characterization of epitopes on the measles virus hemagglutinin protein. Virology 192: 351–354
Hu A, Cattaneo R, Schwartz S, Norrby E (1994) Role of N-linked oligosaccharide chains in the processing and antigenicity of the measles virus hemagglutinin protein. J Gen Virol (in press)
Kornfeld R, Kornfeld S (1985) Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem 54: 631–664
Kreis TE, Lodish HF (1986) Oligomerization is essential for transport of vesicular stomatitis viral glycoprotein to the cell surface. Cell 46: 929–937
Mäkelä MJ, Lund GA, Salmi AA (1989a) Antigenicity of the measles virus haemagglutinin studied by using synthetic peptides. J Gen Virol 70: 603–614
Mäkelä MJ, Salmi AA, Norrby E, Wild TF (1989b) Monoclonal antibodies against measles virus haemagglutinin react with synthetic peptides. Scand J Immunol 30: 225–231
Mottet G, Portner A, Roux L (1986) Drastic immunoreactivity changes between the immature and mature forms of the Sendai virus HN and F0 glycoproteins. J Virol 59: 132–141
Ng DT, Randall RE, Lamb RA (1989) Intracellular maturation and transport of the SV5 hemagglutinin-neuraminidase: specific and transient association with GRP-Bip in the endoplasmic reticulum and extensive internalization from cell surface. J Cell Biol 109: 3273–3289
Norrby E, Hammarskjöld B (1972) Structural components of measles virus. Microbios 5: 17–29
Norrby E, Chen SN, Togashi T, Sheshberadaran H, Johnson KP (1982) Five measles virus antigens demonstrated by use of mouse hybridoma antibodies in productively infected tissue cells. Arch Virol 71: 1–11
Norrby E, Oxman M (1990) Measles virus. In: Fields BN, Knipe DM (eds) Virology, 2nd edn. Raven Press, New York, pp 1013–1044
Pedersen IR, Bog-Hansen TC, Dalsgaord K, Heegaord PMH (1992) Iscom immunization with synthetic peptides representing measles virus hemagglutinin. Virus Res 24: 145–159
Peters J Jr, Davison LK (1982) The biosynthesis of rate serum albumin: in vivo studies on the formation of disulfide bonds. J Biol Chem 257: 8847–8853
Rota JS, Hummel KB, Rota PA, Bellini WJ (1992) Genetic variation of the glycoprotein genes of current wild-type measles isolates. Virology 188: 135–142
Sheshberadaran H, Chen SN, Norrby E (1983) Monoclonal antibodies against five structural components of measles virus. I. Characterization of antigenic determinants on nine strains of measles virus. Virology 128: 341–353
Sheshberadaran H, Norrby E (1986) Characterization of epitopes on the measles virus hemagglutinin protein. Virology 152: 58–65
Sheshberadaran H, Payne GL (1988) Protein antigen-monoclonal antibody contact sites investigated by limited proteolysis of monoclonal antibody-bound antigen: protein “footprinting”. Proc Natl Acad Sci USA 85: 1–5
Tartakoff AM (1986) Temperature and energy dependence of secretory protein transport in the exocrine pancreas. EMBO J 5: 1477–1482
Vidal S, Mottet G, Kolakofsky D, Roux L (1989) Addition of high-mannose sugars must precede disulfide bond formation for proper folding of Sendai virus glycoproteins. J Virol 63: 892–900
Waxham MN, Merz DV, Wolinsky JS (1986) Intracellular maturation of mumps virus hemagglutinin-neuraminidase glycoprotein: conformational changes detected with monoclonal antibodies. J Virol 59: 392–400
Yamada A, Takeuchi K, Hishiyama M (1988) Intracellular processing of mumps virus glycoproteins. Virology 165: 268–273
Yewdell JW, Yellen A, Bachi T (1988) Monoclonal antibodies localize events in the folding, assembly, and intracellular transport of the influenza virus hemagglutinin glycoprotein. Cell 52: 843–852
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hu, A., Kövamees, J. & Norrby, E. Intracellular processing and antigenic maturation of measles virus hemagglutinin protein. Archives of Virology 136, 239–253 (1994). https://doi.org/10.1007/BF01321055
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01321055