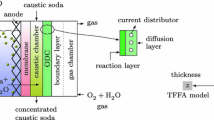
Abstract
Electrochemical production of gases, e.g. Cl2, H2 and O2, is generally carried out in vertical electrolysers with a narrow electrode gap. The evolution of gas bubbles, on one hand, speeds up the mass transport; on the other it increases the solution resistance and also the cell potential. The gas void fraction in the cell increases with increasing height and, consequently, the current density is expected to decrease with increasing height. Insight into the effects of various parameters on the current distribution and the ohmic resistance in the cell is of the utmost importance in understanding the electrochemical processes at gas-evolving electrodes. An example of the described phenomena is the on-site production of hypochlorite by means of a vertical cell. Experiments were carried out with a working electrode consisting of 20 equal segments and an undivided counter electrode. It has been found that the current distribution over the anode is affected by various electrolysis parameters. The current density,j, decreased linearly with increasing distance,h, from the leading edge of the electode. The absolute value of the slope of theI/h straight line increased with increasing average current density and temperature, and with decreasing velocity of the solution, NaCl concentration and interelectrode gap.
Similar content being viewed by others
Abbreviations
- a 1 :
-
constant
- b a :
-
anodic Tafel slope (V)
- b c :
-
cathodic Tafel slope (V)
- B :
-
current distribution factor
- B 0 :
-
current distribution factor att e=0
- c NaCl :
-
sodium chloride concentration (kmol m−3)
- dwt :
-
interelectrode gap (mm)
- h :
-
distance from the leading edge of the segmented electrode (m)
- H :
-
total height of the segmented electrode (m)
- I :
-
current (A)
- I s :
-
current through a segment (A)
- j 0 :
-
exchange current density (kA m−2)
- j av :
-
mean current density (kA m−2)
- j t :
-
current density at the top of the segmented electrode (h=H) (kA m−2)
- j b :
-
current density at the bottom of the segmented electrode (h=0) (kA m−2)
- n s :
-
number of a segment of the segmented electrode from its leading edge
- R s :
-
unit surface resistance of solution (Ω m2)
- R s, b :
-
unit surface resistance of solution at the bottom of the segmented electrode (Ω m2)
- R s, t :
-
unit surface resistance of solution at the top of the segmented electrode (Ω m2)
- t e :
-
time of electrolysis (h)
- T :
-
temperature (K)
- U c :
-
cell voltage (V)
- U 0 :
-
reversible cell voltage (V)
- v 0 :
-
solution flow rate of the bulk solution in the cell at the level of the leading edge of the electrode (m s−1)
- ϱ:
-
resistivity of the solution (Ω m)
- η a :
-
anodic overpotential (V)
- η c :
-
cathodic overpotential (V)
- ε:
-
gas void fraction
- εb :
-
gas void fraction ath=0
- εt :
-
gas void fraction ath=H
References
N. Ibl and H. Vogt, in ‘Comprehensive Treatise of Electrochemistry,’ Plenum Press, New York (1981) Vol. 2, p. 173 ff.
N. Ibl and D. Landolt,Electrochim Acta 15 (1970) 1165.
O. Schwarzer and R. Landsberg,J. Electroanal. Chem. 19 (1968) 405.
J. A. Harrison and Z. A. Khan,J. Electroanal. Chem. 30 (1971) 87.
E. Mueller,Z. Electrochem 5 (1899) 469.
F. Förster, Elektrochemie wässriger Lösungen, J. Ambrosius Barth, Leipzig (1923) 598.
B. B. E. Bongenaar-Schlenter, Thesis, Eindhoven (1985).
J. M. Alice, B. K. Sadanada Rao and G. Venkatamoran,Indian. Chem. Eng. 28 (1986) 49.
J. M. Chin Kwie Joe, L. J. J. Janssen, S. J. van Stralen, J. H. G. Verbunt and W. H. Sluyter,Electrochim Acta 33 (1988) 769.
H. Vogt,Electrochim. Acta 28 (1983) 314.
C. W. M. P. Sillen, Thesis, Eindhoven (1983).
G. R. Heal, A. T. Kuhn and R. B. Lartey,J. Electrochem. Soc. 124 (1977) 1690.
L. J. J. Janssen and E. Barendrecht, in ‘Modern Chlor-Alkali Technology’ (edited by K. Wall), Chichester (1986) Vol. 3, p. 430.
H. M. Gijsbers and L. J. J. Janssen, to be published.
L. Czarnetzki and L. J. J. Janssen,Electrochim. Acta 33 (1988) 561.
R. E. de la Rue and C. W. Tobias,J. Electrochem. Soc. 106 (1959) 827.
E. W. Washburn (editor), ‘International Critical Tables,’ McGraw-Hill, New York (1929).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Czarnetzki, L.R., Janssen, L.J.J. Electrode current distribution in a hypochlorite cell. J Appl Electrochem 19, 630–636 (1989). https://doi.org/10.1007/BF01320637
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01320637