Summary
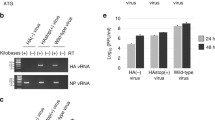
Virus-specific macromolecule synthesis has been examined in BHK cells infected with Ross River virus. Unpassaged virus (R-0) and tenth-passage virus (R-10) have been compared. In infected cells R-0 generates i) 45S, 38S, 33S and 26S viral RNAs, ii) virus-specific precursor polypeptides of mol. wt. 127,000, 95,000 and 61,000 and iii) viral envelope proteins (mol. wts. 52,000 and 49,000) and nucleocapsid protein (mol. wt. 32,000). Thus in terms of virus-specific RNA and polypeptide synthesis, the replication of standard RRV is analogous to that of Semliki Forest virus and Sindbis virus.
R-10 interferes with the replication of standard Ross River virus and generates large amounts of 19S and 24S defective RNA species; 45S and 26S RNA synthesis was not markedly affected. Defective RNAs are associated with RNAse-sensitive, 50S cytoplasmic particles which contain a variety of (mainly host) proteins but no nucleocapsid protein. No evidence for translation of defective RNAs was obtained.
R-10 infection is also characterized by a relatively early shut down of host protein synthesis and by a reduction in virus-specific polypeptide synthesis and nucleocapsid formation. The data suggest that defective Ross River virus interferes primarily at the translational level.
Similar content being viewed by others
References
Aviv, H., Leder, P.: Purification of biologically active globin messenger RNA by chromatography on oligo-thymidylic acid-cellulose. Proc. Nat. Acad. Sci. (U.S.A.)69, 1408–1412 (1972).
Baltimore, D., Huang, A. S.: Isopycnic separation of subcellular components from poliovirus-infected and normal HeLa cells. Science162, 572–574 (1968).
Bonner, W. M., Laskey, R. A.: A film detection method for tritium-labelled proteins and nucleic acids in poly-acrylamide gels. Europ. J. Biochem.46, 83–88 (1974).
Bruton, C. J., Kennedy, S. I. T.: Defective interfering particles of Semliki Forest virus: Structural differences between standard virus and defective-interfering particles. J. gen. Virol.31, 383–395 (1976).
Bruton, C. J., Porter, A., Kennedy, S. I. T.: Defective-interfering particles of Semliki Forest virus: Intracellular events during interference. J. gen. Virol.31, 397–416 (1976).
Dalgarno, L., Martin, E. M., Liu, S.-L., Work, T. S.: Characterization of the products formed by RNA polymerases of cells infected with encephalomyocarditis virus. J. mol. Biol.15, 77–91 (1966).
Davey, M. W., Dalgarno, L.: Semliki Forest virus replication in culturedAedes albopictus cells: Studies on the establishment of persistence. J. gen. Virol.24, 453–463 (1974).
Doherty, R. L., Whitehead, R. H., Gorman, B. M., O'Gower, A. K.: The isolation of a third group A arbovirus in Australia, with preliminary observations on its relationship to epidemic polyarthritis. Aust. J. Science26, 183–184 (1963).
Eaton, B. T., Faulkner, P.: Altered pattern of viral RNA synthesis in cells infected with standard and defective Sindbis virus. Virology51, 85–93 (1973).
Garoff, H., Simons, K., Renkonen, O.: Isolation and characterization of the membrane proteins of Semliki Forest virus. Virology61, 493–504 (1974).
Guild, G. M., Stollar, V.: Defective interfering particles of Sindbis virus. III. Intracellular viral RNA species in chick embryo cell cultures. Virology67, 24–41 (1975).
Huang, A. S., Defective interfering viruses. Ann. Rev. Microbiol.27, 101–117 (1973).
Johnston, R. E., Tovell, D. R., Brown, D. T., Faulkner, P.: Interfering passages of Sindbis virus: Concomitant appearance of interference, morphological variants and truncated viral RNA. J. Virol.16, 951–958 (1975).
Kaluza, G.: Early synthesis of Semliki Forest virus-specific proteins in infected chicken cells. J. Virol.19, 1–12 (1976).
Kennedy, S. I. T.: Sequence relationships between the genome and the intracellular RNA species of standard and defective-interfering Semliki Forest virus. J. mol. Biol.108, 491–511 (1976).
Kennedy, S. I. T., Bruton, C. J., Weiss, B., Schlesinger, S.: Defective interfering passages of Sindbis virus: Nature of the defective virion RNA. J. Virol.19, 1034–1043 (1976).
Laemmli, U. K.: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature227, 680–685 (1970).
Levin, J. G., Ramseur, J. M., Grimley, P. M.: Host effect on arbovirus replication: Appearance of defective interfering particles in murine cells. J. Virol.12, 1401–1406 (1973).
Mims, C. A., Murphy, F. A., Taylor, W. P., Marshall, I. D.: Pathogenesis of Ross River virus infection in mice. I. Ependymal infection, cortical thinning, and hydrocephalus. J. inf. Dis.127, 121–128 (1973).
Murphy, F. A., Taylor, W. P., Mims, C. A., Marshall, I. D.: Pathogenesis of Ross River virus infection in mice. II. Muscle, heart and brown fat lesions. J. inf. Dis.127, 129–138 (1973).
Raghow, R. S., Grace, T. D. C., Filshie, B. K., Bartley, W., Dalgarno, L.: Ross River virus replication in cultured mosquito and mammalian cells: Virus growth and correlated ultrastructural changes. J. gen. Virol.21, 109–122 (1973).
Schlesinger, S., Schlesinger, M., Burge, B. W.: Defective virus particles from Sindbis virus. Virology48, 615–617 (1972).
Shenk, T. E., Stollar, V.: Defective-interfering particles of Sindbis virus. I. Isolation and some chemical and biological properties. Virology53, 162–173 (1973).
Shine, J., Dalgarno, L.: Occurrence of heat-dissociable ribosomal RNA in insects: The presence of three polynucleotide chains in 26S RNA from culturedAedes aegypti cells. J. mol. Biol.75, 57–72 (1973).
Simmons, D. T., Strauss, J. H.: Replication of Sindbis virus. V. Polyribosomes and mRNA in infected cells. J. Virol.14, 552–559 (1974).
Weiss, B., Schlesinger, S.: Defective interfering passages of Sindbis virus: Chemical composition, biological activity and mode of interference. J. Virol.12, 862–871 (1973).
Weiss, B., Goran, D., Cancedda, R., Schlesinger, S.: Defective interfering passages of Sindbis virus: Nature of the intracellular defective viral RNA. J. Virol.14, 1189–1198 (1974).
Westaway, E. G., Reedman, B. M.: Proteins of the group B arbovirus Kunjin. J. Virol.4, 688–693 (1969).
Author information
Authors and Affiliations
Additional information
With 11 Figures
Rights and permissions
About this article
Cite this article
Martin, J.H.B., Weir, R.C. & Dalgarno, L. Replication of standard and defective Ross River virus in BHK cells: Patterns of viral RNA and polypeptide synthesis. Archives of Virology 61, 87–103 (1979). https://doi.org/10.1007/BF01320594
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01320594