Summary
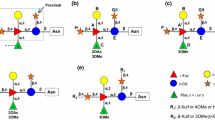
Benzhydrazone is a bis-amidinohydrazone derivative which specifically hinders the formation of herpes simplex virus (HSV) glycoproteins. In this study we present some structural features of the oligosaccharide chains of herpesvirus glycoproteins synthesized in cells incubated with the inhibitor. Gel filtration analysis of glycopeptides, obtained through exhaustive pronase-digestion of infected cells after a long or a short labeling with14C-glucosamine, showed that benzhydrazone reduced the appearance of glycopeptides of all the size-classes, including the mannose-rich glycopeptide with an approximate molecular weight of 1500. The same per cent of label was released from both untreated and benzhydrazone-treated cells after digestion with endo-β-N-acetylglucosaminidase H, an enzyme which cleaves between the N-acetylglucosamine residues in large highmannose type oligosaccharides. This indicates that the relative amount of glycoproteins sensitive to this enzyme did not differ in the two kinds of samples. PAGE analysis confirmed that the same glycoproteins were digested in both samples. They were gA, pgC, and pgD, which therefore contain high-mannose type oligosaccharides. It is concluded that benzhydrazone hinders carbohydrate addition to herpesvirus proteins at an early step.
Similar content being viewed by others
References
Abbondanza, A., Franceschi, C., Licastro, F., Serafini-Cessi, F.: Properties of a glycopeptide isolated from human Tamm-Horsfall glycoprotein. Interaction with leucoagglutinin and anti-(human Tamm-Horsfall glycoprotein) antibodies. Biochem. J.187, 525–528 (1980).
Afonso, A., Charlwood, P. A., Marshall, R. D.: Isolation and characterization of glycopeptides from digests of human Tamm-Horsfall glycoprotein. Carbohydr. Res.89, 309–320 (1981).
Arima, T., Spiro, R. G.: Studies on the carbohydrate unit of thyroglobulin. Structure of the mannose-N-acetylglucosamine unit (unit A) of the human and calf proteins. J. biol. Chem.247, 1836–1848 (1972).
Campadelli-Fiume, G., Sinibaldi-Vallebona, P., Cavrini, V., Mannini-Palenzona, A.: Selective inhibition of herpes simplex virus glycoprotein synthesis by a benz-amidinohydrazone derivative. Arch. Virol.66, 179–181 (1980).
Cavrini, V., Gatti, R., Roveri, P., Giovanninetti, G., Mannini-Palenzona, A., Baserga, M.: Antiviral compounds. XII. Synthesis andin vitro antiherpetic activity of some cyclic α- and β-diketone bis-amidinohydrazones. Eur. J. med. Chem.14, 343–346 (1979).
Cohen, G. H., Long, D., Eisenberg, R. J.: Synthesis and processing of glycoproteins gD and gC of Herpes Simplex Virus type 1. J. Virol.36, 429–439 (1980).
Eberle, R., Courtney, R. C.: gA and gB glycoproteins of herpes simplex virus type 1: two forms of a single polypeptide. J. Virol.36, 665–675 (1980).
Eisenberg, R. J., Hydrean-Stern, C., Cohen, G. H.: Structural analysis of precursor and product forms of type-common envelope glycoprotein D (CP-1 antigen) of herpes simplex virus type 1. J. Virol.31, 608–620 (1979).
Ejercito, P. L., Kieff, E. D., Roizman, B.: Characterization of herpes simplex strains differing in their effects on social behaviour of infected cells. J. gen. Virol.2, 357–364 (1968).
Fletcher, A. P., Marks, G. S., Marshall, R. D., Neuberger, A.: Carbohydrates in protein. 5. Procedures for the isolation of glycopeptides from hen's-egg albumin and their oxidation by periodate. Biochem. J.87, 265–273 (1963).
Heifetz, A., Keenan, R. W., Elbein, A. D.: Mechanism of action of tunicamycin on the UDP-GlcNac: dolichyl-phosphate GlcNAc-1-phosphate transferase. Biochemistry18, 2186–2192 (1979).
Honess, R. W., Roizman, B.: Proteins specified by herpes simplex virus. XIII. Glycosylation of viral polypeptides. J. Virol.16, 1308–1326 (1975).
Hunt, L. A., Etchison, J. R., Summers, D.: Oligosaccharide chains are trimmed during synthesis of the envelope glycoprotein of vesicular stomatitis virus. Proc. Natl. Acad. Sci. U.S.A.75, 754–758 (1978).
Ito, S., Muramatsu, T., Kobata, A.: Endo-β-N-acetylglucosaminidases acting on carbohydrate moieties of glycoproteins: purification and properties of the two enzymes with different specificities fromClostridium perfringens. Arch. Biochem. Biophys.171, 78–86 (1975).
Klenk, H. D., Rott, R.: Cotranslational and posttranslational processing of viral glycoproteins. Curr. Top. Microbiol. Immunol.90, 19–48 (1980).
Klenk, H. D., Wöllert, W., Rott, R., Scholtissek, C.: Association of influenza virus proteins with cytoplasmic fractions. Virology57, 28–41 (1974).
Lowry, O. H., Rosebrough, W. J., Farr, A. L., Randall, R. J.: Protein measurement with the folin reagent. J. biol. Chem.193, 265–275 (1951).
Nakamura, K., Compans, R. W.: Effects of glucosamine, 2-deoxyglucose, and tunicamycin on glycosylation, sulfation, and assembly of influenza viral proteins. Virology84, 303–319 (1978).
Nomoto, M., Narahashi, Y.: A proteolytic enzyme of Streptomyces griseus. I. Purification of a protease of Streptomyces griseus. J. Biochem.46, 653–667 (1959).
Norrild, B.: Immunochemistry of herpes simplex virus glycoproteins. Curr. Top. Microbiol. Immunol.90, 67–106 (1980).
Olofsson, S., Blomberg, J.: Studies on glycopeptides of herpes simplex virus infected cells. Arch. Virol.55, 293–304 (1977).
Olofsson, S., Jeansson, S., Lycke, E.: Unusual lectin-binding properties of a herpes simplex virus type 1-specific glycoprotein. J. Virol.38, 564–570 (1981).
Olofsson, S., Lycke, E.: Glucosamine metabolism of herpes simplex virus infected vcells. Inhibition of glycosylation by tunicamycin and 2-deoxy-D-glucose. Arch. Virol.65, 201–209 (1980).
Pizer, L. I., Cohen, G. H., Eisenberg, R. J.: Effect of tunicamycin on herpes simplex virus glycoproteins and infectious virus production. J. Virol.34, 142–153 (1980).
Robbins, P. W., Hubbard, S. C., Turco, J. S., Wirth, D. F.: Proposal for a common oligosaccharide intermediate in the synthesis of membrane glycoproteins. Cell12, 893–900 (1977).
Schwartz, R. T., Klenk, H. D.: Inhibition of glycosylation of influenza virus hemagglutinin. J. Virol.14, 1023–1034 (1974).
Sefton, B. M., Keegstra, K.: Glycoproteins of Sindbis virus: preliminary characterization of the oligosaccharides. J. Virol.14, 522–530 (1974).
Spear, P. G.: Membrane proteins specified by herpes simplex viruses. I. Identification of four glycoprotein precursors and their products in type 1-infected cells. J. Virol.17, 991–1008 (1976).
Staneloni, R. J., Leloir, L. F.: The biosynthetic pathway of the asparagine-linked oligosaccharides of glycoproteins. Trends biochem. Sci.4, 65–67 (1979).
Tabas, I., Schlesinger, S., Kornfeld, S.: Processing of the high mannose oligosaccharide to form complex type oligosaccharides on the newly synthesized polypeptides of the vesicular stomatitis virus G protein and the IgG heavy chain. J. biol. Chem.253, 716–722 (1978).
Takatsuki, A., Tamura, G.: Effect of tunicamycin on the synthesis of macromolecules in cultures of chick embryo fibroblasts infected with Newcastle disease virus. J. Antib. (Tokyo)24, 785–794 (1971).
Tarentino, A. L., Maley, F.: Purification and properties of an endo-β-N-acetylglucosaminidase fromStreptomyces griseus. J. biol. chem.249, 811–816 (1974).
Tkacz, J. S., Lampen, J. O.: Tunicamycin inhibition of polyisoprenil N-acetylglucosaminyl pyrophosphate formation in calf liver microsomes. Biochem. biophys. Res. Commun.65, 248–257 (1975).
Warren, L.: The thiobarbituric acid assay of sialic acids. J. biol. Chem.234, 1971–1975 (1959).
Author information
Authors and Affiliations
Additional information
With 5 Figures
Rights and permissions
About this article
Cite this article
Serafini-Cessi, F., Campadelli-Fiume, G. Studies on benzhydrazone, a specific inhibitor of herpesvirus glycoprotein synthesis. Size distribution of glycopeptides and endo-β-N-acetylglucosaminidase-H treatment. Archives of Virology 70, 331–343 (1981). https://doi.org/10.1007/BF01320248
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01320248