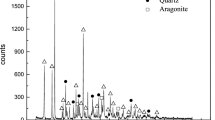
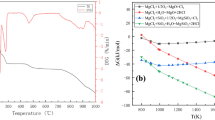
Conclusions
Natural and caustic magnesite can be concentrated by chemical methods to an MgO content of 98%. An analysis of these methods showed that they are technically feasible and economically advantageous.
The hydrochloric acid and ammonium methods of beneficiation give not only pure magnesia but also magnesia-based composition materials with a regulatable components proportion including materials in the MgO-CaO system. A high degree of homogenization of the components can be achieved in these processes without the use of mixing equipment.
In the chemical beneficiation of magnesite the sinterability of the magnesia can be regulated to give high-density sintered powders with the required grain-size distribution.
It will be necessary to expand research in the field of the chemical beneficiation of raw materials including magnesium-containing rocks, and to develop methods of producing composition materials and regulating the sinterability of pure oxides.
Similar content being viewed by others
Literature cited
A. K. Karklit, Yu. A. Polonskii, K. K. Strelov, et al., in: Refractories Production, Trans. All-Union Inst. Refract. [in Russian], No. 2(45), VIO, Leningrad (1974), pp. 3–26.
Proceeding of the International Symposium: “Technical Progress in the Ferrous Metallurgy of the CMEA Countries and Yugoslavia” [in Russian], SÉV, Moscow (1973).
N. I. Baranovskii, S. I. Kropan'ev, R. P. Yagovkina, et al., in: Trans. Ural Inst. of Minerals Beneficiation and Processing [in Russian], No. 17, Uralmekhanobr, Sverdlovsk (1970), pp. 63–69.
H. Hoppe and B. Maurer, Chemische Technik, No. 5, 257–260 (1966).
Yu. Ya. Kaganovich, A. G. Zlobinskii, G. N. Sargayan, et al., in: Problems in the Technology of Magnesia Products [in Russian], GIPKh, Leningrad (1973), pp. 107–114.
M. V. Pozin, Technology of Mineral Salts [in Russian], Pt. 1, Khimiya, Leningrad (1970).
B. A. Shoikhet, L. V. Sologubenko, and A. N. Kolesova, in: Problems in the Technology of Magnesia Products [in Russian], GIPKh, Leningrad (1973), pp. 87–98.
A. S. Berezhnoi, in: Proceedings of the Third All-Union Conference on Refractory Materials [in Russian], Izd. Akad. Nauk SSSR, Moscow-Leningrad (1947), pp. 14–21.
W. C. Gilpin, Refractories J., No. 3, 68–82 (1969).
Author information
Authors and Affiliations
Additional information
Translated from Ogneupory, No. 2, pp. 17–23, February, 1977.
Rights and permissions
About this article
Cite this article
Gavrish, D.I., Strelov, K.K., Galkin, Y.M. et al. Chemical methods of beneficiating magnesite. Refractories 18, 83–89 (1977). https://doi.org/10.1007/BF01319656
Issue Date:
DOI: https://doi.org/10.1007/BF01319656