Summary
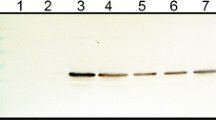
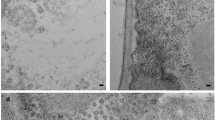
During the development of systemic mosaic symptoms in tobacco mosaic virus (TMV)-infected tobacco, the viral non-structural 126-kDa-protein was present among the chromatin-associated proteins in fractionated leaf homogenates [Van Telgen HJ et al. (1984) Virology 143: 612–616]. Using an antiserum raised against a fusion protein of β-galactosidase and part of the 126-kDa-protein of TMV, this viral protein was detected by immunoelectron microscopy in X-bodies in infected tissue. No labelling of nuclei was apparent. However, in embedded purified nuclear preparations from systemically infected leaves amorphous structures, most likely X-bodies, were present and specifically labelled. In contrast, using antibodies against tobacco histones, only nuclei were labelled. Antibodies against viral coat protein labelled crystalline virus inclusions in the cytoplasm and did not react with nuclei. Light microscopic analysis indicated that X-bodies were almost always associated with nuclei. Thus, the presence of X-bodies in nuclear preparations appeared to result from adherence of the X-bodies to the nuclei.
Similar content being viewed by others
References
Ahlquist P, Strauss EG, Rice CM, Strauss JH, Haseloff J, Zimmern D (1985) Sindbis virus proteins nsP 1 and nsP 2 contain homology to nonstructural proteins from several RNA plant viruses. J Virol 53: 536–542
Christie RG, Edwardson JK (1977) Light and electron microscopy of plant virus inclusions. Florida Agricultural Experiment Stations Monograph Series 9: 11–27
Commoner B, Yamada M (1955) The non virus proteins associated with tobacco mosaic virus. J Gen Physiol 38: 459–473
Deom CM, Oliver MJ, Beachy RN (1987) The 30-kilodalton gene product of tobacco mosaic virus potentiates virus movement. Science 237: 389–394
Esau K, Cronshaw J (1967) Relation of tobacco mosaic virus to the host cells. J Cell Biol 33: 665–678
Fraenkel-Conrat H (1957) Degradation of tobacco mosaic virus with acetic acid. Virology 4: 1–4
Franssen H, Goldbach R, Broekhuysen M, Moerman M, Van Kammen A (1982) Expression of middle component RNA of cowpea mosaic virus: in vitro generation of a precursor to both capsid proteins by a bottom component RNA-encoded protease from infected cells. J Gen Virol 41: 8–17
Goelet P, Lomonosoff GP, Butler PJG, Akam ME, Gait MJ, Karn J (1982) Nucleotide sequence of tobacco mosaic virus RNA. Proc Natl Acad Sci USA 79: 5818–5822
Goldstein B (1926) A cytological study of the leaves and growing points of healthy and mosaic diseased tobacco plants. Bull Torrey Bot Club 53: 499–599
Grayeb J, Kimura H, Takahara M, Hsiung H, Masui Y, Inoue M (1984) Secretion cloning vectors in Escherichia coli. EMBO J 3: 2437–2442
Hills GJ, Plaskitt KA, Young ND, Dunigan DD, Watts JW, Wilson TMA, Zaitlin M (1987) Immunogold localization of the intracellular sites of structural and non-structural tobacco mosaic virus proteins. Virology 161: 488–496
Huang WM, Gibson SJ, Facer P, Gu J, Polak JM (1983) Improved section adhesion for immunocytochemistry using molecular weight polymers of L-lysine as a slide coating. Histochemistry 77: 275–277
Jackson RJ, Hunt T (1983) Preparation and use of nuclease-treated rabbit reticulocyte lysates for the translation of eukaryotic messenger RNA. Methods Enzymol 96: 50–74
Kamer G, Argos P (1984) Primary structural comparison of RNA-dependent polymerases from plant, animal and bacterial viruses. Nucleic Acids Res 12: 7269–7282
Knight CA (1975) Chemistry of viruses. Springer, Wien New York, pp 8–25
Laemmli UK (1970) Cleavage of structural proteins during assembly of the head of bacteriophage T 4. Nature 277: 680–685
Leonard DH, Zaitlin M (1982) A temperature-sensitive strain of tobacco mosaic virus defecive in cell-to-cell movement generates an altered viral-coded protein. Virology 117: 416–424
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, New York, p 440
Ohno N, Takamatsu N, Meshi T (1983) Single amino acid substitution in 30k protein of TMV defective in virus transport function. Virology 131: 255–258
Pelham HRB (1979) Synthesis and proteolytic processing of cowpea mosaic virus proteins in reticulocyte lysates. Virology 96: 463–477
Reynolds ES (1963) The use of lead citrate at high pH as an electron opaque stain in electron microscopy. J Cell Biol 17: 208–212
Rezelman G, Goldbach R, Van Kammen A (1980) Expression of bottom component RNA of cowpea mosaic virus in cowpea protoplasts. J Virol 36: 366–373
Romaine CP, Zaitlin M (1978) RNA-dependent RNA polymerases in uninfected and tobacco mosaic virus-infected tobacco leaves: viral-induced stimulation of a host polymerase activity. Virology 86: 241–253
Saito T, Watanabe Y, Meshi T, Okada Y (1986) Preparation of antibodies that react with the large non-structural proteins of tobacco mosaic virus by using Escherichia coli expressed fragments. Mol Gen Genet 205: 82–89
Saito T, Hosakawa D, Meshi T, Okada Y (1987) Immunocytochemical localization of the 130k and 180k (putative replicase components) of tobacco mosaic virus. Virology 160: 477–481
Scalla R, Romaine P, Asselin A, Rigaud J, Zaitlin M (1978) An in vivo study of a structural polypeptide synthesized upon TMV infection and its identification with a polypeptide synthesized in vitro from TMV RNA. Virology 91: 182–183
Slot JW, Geuze HJ (1981) Sizing of protein A-colloidal gold probes for immunoelectron microscopy. J Cell Biol 90: 533–536
Takahashi WN, Ishii M (1953) A macromolecular protein associated with tobacco mosaic virus infection: its isolation and properties. Am J Bot 40: 85–90
Towill LE, Nooden LD (1973) An electrophoretic analysis of the acid soluble chromosomal proteins from different organs of maize seedlings. Plant Cell Physiol 14: 851–863
Van den Berg BM, Wijsman HWJ (1981) Genetics of the peroxidase isoenzymes in Petunia. I. Organ specificity and general aspects of the peroxidase isoenzymes. Theor Appl Genet 60: 71–76
Van Lent JWM, Verduin BJM (1985) Specific gold-labelling of antibodies bound to plant viruses in mixed suspensions. Neth J Plant Pathol 91: 205–213
Van Lent JWM, Verduin BJM (1986) Detection of viral protein and particles in thin sections of infected plant tissue using immunogold labelling. In: Jones RAC, Torrance L (eds) Developments in applied biology, vol 1, developments and applications in virus testing. Assoc Applied Biologists, Wellesbourne, pp 193–211
Van Lent JWM, Verduin BJM (1987) Detection of viral antigen in semi-thin sections of plant tissue by immunogold-silver staining and light microscopy. Neth J Plant Pathol 93: 261–272
Van Loon LC (1987) Disease induction by plant viruses. Adv Virus Res 33: 205–255
Van Loon LC, Van Kammen A (1970) Polyacrylamide disc electrophoresis of the soluble leaf proteins from Nicotiana tabacum var. ‘Samsun’ and ‘Samsun NN’. II Changes in protein constitution after infection with tobacco mosaic virus. Virology 40: 199–211
Van Telgen HJ, Van Loon LC (1983) Isolation and electrophoretic analysis of chromatin-associated proteins from virus-infected tobacco leaves. Z Pflanzenphysiol 112: 171–180
Van Telgen HJ, Goldbach RW, Van Loon LC (1984) The 126.000 molecular weight protein of tobacco mosaic virus is associated with host chromatin in mosaic-diseased tobacco plants. Virology 143: 612–616
Van Telgen HJ, Van der Zaal EJ, Van Loon LC (1985) Some characteristics of the association of the 116kD protein with host chromatin in tobacco leaves infected with tobacco mosaic virus. Physiol Plant Pathol 26: 99–109
Whenham RJ, Fraser RSS, Snow A (1985) Tobacco mosaic virus-induced increase in abscisic acid concentration in tobacco leaves: intracellular location and relationship to symptom severity and to extent of virus multiplication. Physiol Plant Pathol 26: 379–387
Zabel P, Moerman M, Van Straaten F, Goldbach R, Van Kammen A (1982) Antibodies against the genome-linked protein VPg of cowpea mosaic virus recognize a 60.000 dalton precursor polypeptide. J Virol 41: 1083–1088
Zimmern D, Hunter T (1983) Point mutation in the 30k reading frame of TMV implicated in temperature sensitive assembly and local lesion spread of mutant Ni 2519. EMBO J 2: 1893–1900
Zimmern D (1989) Evolution of RNA viruses. In: Hollant J, Doningo E, Ahlquist P (eds) RNA genetic, vol 2. CRC Press, Boca Raton (in press)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wijdeveld, M.M.G., Goldbach, R.W., Verduin, B.J.M. et al. Association of viral 126kDa protein-containing X-bodies with nuclei in mosaic-diseased tobacco leaves. Archives of Virology 104, 225–239 (1989). https://doi.org/10.1007/BF01315545
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01315545