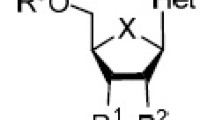Summary
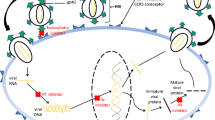
The human immunodeficiency virus 1 (HIV-1) codes for a proteinase that cuts viral proteins at specific sites. We have tested 13 modified oligopeptides related to these cleavage sites to see if they inhibit viral replication. To indicate whether a decrease in replication could be due to a general inhibition of cell metabolism, we also measured the effect of the peptides on cellular protein synthesis. Three of the peptides tested (Ac-Gln-Asn-Sta-Val-NH2, Ac-Gln-Asn-Sta-Val-Val-NH2, and Ac-Glu-Asn-Sta-Ile-NH2) inhibited HIV-1 replication at concentrations that did not inhibit protein synthesis. Ac-Gln-Asn-Sta-Val-NH2 was the most potent, causing an approximately 40% decrease in viral replication, measured as the synthesis of HIV-1 antigens and the formation of infectious particles.
Similar content being viewed by others
References
Anwer MK, Spatola AF (1980) An advantageous method for the rapid removal of hydrogenolysable protecting groups under ambient conditions; synthesis of leucine-enkephalin. Synthesis 12: 929–932
Billich S, Knoop M-T, Hansen J, Strop P, Sedlacek J, Mertz R, Moelling K (1988) Synthetic peptides as substrates and inhibitors of human immune deficiency virus-1 protease. J Biol Chem 263: 17905–17908
Blundell TL, Cooper J, Foundling SI, Jones DM, Atrash B, Szelke M (1987) On the rational design of renin inhibitors: X-ray studies of aspartic proteinases complexed with transition-state analogues. Biochemistry 26: 5585–5590
Crawford S, Goff SP (1985) A deletion mutation in the 5′ part of the pol gene of Moloney murine leukemia virus blocks proteolytic processing of the gag and pol polyproteins. J Virol 53: 899–907
Darke PL, Nutt RF, Brady SF, Garsky VM, Ciccarone TM, Leu C-T, Lumma PK, Freidinger RM, Veber DF, Sigao IS (1988) HIV-1 protease specificity of peptide cleavage is sufficient for processing of gag and pol polyproteins. Biochem Biophys Res Commun 156: 297–303
Dreyer GB, Metcalf BW, Tomaszek TA, Carr TJ, Chandler AC, Hyland L, Fakhoury SA, Magaard VW, Moore ML, Strickler JE, Debouck C, Meek TD (1989) Inhibition of human immunodeficiency virus 1 protease in vitro: rational design of substrate analogue inhibitors. Proc Natl Acad Sci USA 86: 9752–9756
Grinde B, Hungnes O, Tjøtta E (1989) The proteinase inhibitor pepstatin A inhibits formation of reverse transcriptase in H9 cells infected with human immunodeficiency virus 1. AIDS Res Hum Retrovir 5: 269–274
Holladay MW, Salituro FG, Rich DH (1987) Synthetic and enzyme inhibition studies of pepstatin analogues containing hydroxyethylene and ketomethylene dipeptide isosteres. J Med Chem 30: 374–383
Katoh I, Yasunaga T, Ikawa Y, Yoshinaka Y (1987) Inhibition of retroviral protease activity by an aspartyl proteinase inhibitor. Nature 329: 654–657
Katoh I, Yoshinaka, Y, Rein A, Shibuya M, Odaka T, Oroszlan S (1985) Murine leukemia virus muration: protease region required for conversion from “immature” to “mature” core form and for virus infectivity. Virology 145: 280–292
Kleinert HD, Luly JR, Marcotte PA, Perun TJ, Plattner JJ, Stein H (1988) Renin inhibitors. Improvements in the stability and biological activity of small peptides containing novel Leu-Val replacements. FEBS Letts 230: 38–42
Kohl NE, Eimin EA, Schleif WA, Davis LJ, Heimbach JC, Dixon RAF, Scolnick EM, Sigal IS (1988) Active human immunodeficiency virus protease is required for viral infectivity. Proc Natl Acad Sci USA 85: 4686–4690
Kräusslich H-G, Schneider H, Zybarth G, Carter CA, Wimmer E (1988) Processing of in vitro-synthesized gag precursor proteins of human immunodeficiency virus (HIV) type 1 by HIV proteinase generated inEscherichia coli. J Virol 62: 4393–4397
Kräusslich H-G, Wimmer E (1988) Viral proteinases. Annu Rev Biochem 57: 701–754
Le Grice SFJ, Mills J, Mous J (1988) Active site mutagenesis of the AIDS virus protease and its alleviation by trans complementation. EMBO J 7: 2547–2553
Loeb DD, Hutchison CA III, Edgell MH, Farmerie WG Swanstrom R (1989) Mutational analysis of human immunodeficiency virus type 1 protease suggests functional homology with aspartic proteinases. J Virol 63: 111–121
Meek TD, Dayton BD, Metcalf BW, Dreyer GB, Strickler JE, Gorniak JG, Rosenberg M, Moore ML, Magaard VW, Debouck C (1989) Human immunodeficiency virus 1 protease expressed inEscherichia coli behaves as a dimeric aspartic protease. Proc Natl Acad Sci USA 86: 1841–1845
Meek TD, Lambert DM, Dreyer GB, Carr TJ, Tomaszek TA, Moore ML, Strickler JE, Debouck C, Hyland LJ, Matthews TJ, Metcalf BW, Petteway SR (1990) Inhibition of HIV-1 protease in infected T-lymphocytes by synthetic peptide analogues. Nature 343: 90–92
Miller M, Schneider J, Sathyanarayana BK, Toth MV, Marshall GR, Clawson L, Selk L, Kent SBH, Wlodawer A (1989) Structure of complex of synthetic HIV-1 protease with a substrate-based inhibitor at 2.3 A resolution. Science 246: 1149–1152
Navia MA, Fitzgerald PMD, McKeever BM, Leu C-T, Heimbach JC, Herber WK, Sigal IS, Darke PL, Springer JP (1989) Three-dimensional structure of aspartyl protease from human immunodeficiency virus HIV-1. Nature 337: 615–620
Richards AD, Roberts R, Dunn BM, Graves MC, Kay J (1989) Effective blocking of HIV-1 proteinase activity by characteristic inhibitors of aspartic proteinases. FEBS Lett 247: 113–117
Salituro FG, Agarwal N, Hofmann T, Rich DH (1987) Inhibition of aspartic proteinases by peptides containing lysine and ornithine side-chain analogues of statine. J Med Chem 30: 286–295
Seelmeier S, Schmidt H, Turk V, von der Helm K (1988) Human immunodeficiency virus has an aspartic-type protease that can be inhibited by pepstatin A. Proc Natl Acad Sci USA 85: 6612–6616
Skalka AM (1989) Retroviral proteases: first glimpses at the anatomy of a processing machine. Cell 56: 911–913
Tjøtta E, Hungnes O, Grinde B (1990) An assay for quantifying infectious HIV particles. J Virol Methods 27: 169–174
Trono D, Feinberg MB, Baltimore D (1990) HIV-1 gag mutants can dominantly interfere with the replication of the wild-type virus. Cell 59: 113–120
Weber IT, Miller M, Jaskolski M, Leis J, Skalka AM, Wlodawer A (1989) Molecular modeling of the HIV-1 protease and its substrate binding site. Science 243: 928–931
Wlodawer A, Miller M, Jaskolski M, Sathyanarayana BK, Baldwin E, Weber IT, Selk LM, Clawson L, Schneider J, Kent SBH (1989) Conserved folding in retroviral proteases: crystal structure of a synthetic HIV-1 protease. Science 245: 616–621
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Grinde, B., Hungnes, O. & Tjøtta, E. Modified oligopeptides designed to interact with the HIV-1 proteinase inhibit viral replication. Archives of Virology 114, 167–173 (1990). https://doi.org/10.1007/BF01310746
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01310746