Summary
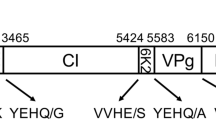
The 3′-terminal 1902 nucleotides of the tobacco vein-banding mosaic virus (TVBMV) were cloned and sequenced. The sequence contains a large open reading frame of 572 amino acid residues followed by a non-coding region of 183 nucleotides which terminates in a polyadenylate tract. The TVBMV coat protein is processed from a larger polyprotein by cleavage at a Gln/Gly dipeptide, which is followed closely by the aphid transmission DAG triplet. Translation of the nucleotide sequence yielded a protein of 271 amino acid residues with a calculated Mr of 30 347. The amino acid and nucleotide sequences of the TVBMV coat protein and 3′ non-coding region were compared to 33–35 other virus sequences. Percent sequence identity of the amino acid and nucleotide sequences were in the range expected from comparison of distinct viruses. Phylogenetic analyses were also performed to determine the relationship of TVBMV to other well-characterized viruses. These studies indicate that TVBMV should be regarded as a distinct member of the potyvirus group.
Similar content being viewed by others
References
Allison RF, Sorenson JC, Kelly ME, Armstrong FB, Dougherty WG (1985) Sequence determination of the capsid protein gene and flanking regions of tobacco etch virus: Evidence for synthesis and processing of a polyprotein in potyvirus genome expression. Proc Natl Acad Sci USA 82: 3969–3972
Atreya CD, Raccah B, Pirone TP (1990) A point mutation in the coat protein abolishes aphid transmissibility of a potyvirus. Virology 178: 161–165
Brand RJ, Burger JT, Rybicki EP (1993) Cloning, sequencing, and expression inEscherichia coli of the coat protein gene of a new potyvirus infecting South African passiflora. Arch Virol 128: 29–41
Brunt AA (1992) General properties of potyviruses. In: Barnett OW (ed) Potyvirus taxonomy. Springer, Wien New York, pp 3–16 (Arch Virol [Suppl] 5)
Burger JT, Brand RJ, Rybicki EP (1990) The molecular cloning and nucleotide sequencing of the 3'-terminal region of ornithogalum mosaic virus. J Gen Virol 71: 2527–2534
Cassidy B, Sherwood JL, Nelson RS (1993) Cloning of the capsid protein gene from a blotch isolate of peanut stripe virus. Arch Virol 128: 287–297
Chin, WT (1966) A survey of tobacco mosaic viruses in central Taiwan. J Agric Assoc China 55: 85–88
Collins RF, Leclerc D, AbouHaidar MG (1993) Cloning and nucleotide sequence of the capsid protein and the nuclear inclusion portein (NIb) of potato virus A. Arch Virol 128: 135–142
Dayhoff MO, Schwarz RM, Orcutt BC (1979) A model of evolutionary change in proteins. In: Dayhoff MO (eds) Atlas of protein sequence and structure. Natl Biomed Res Found, Washington, pp 1–8
Dekker EL, Derks A, Asjes CJ, Lemmers M, Bol JF, Langeveld SA (1993) Characterization of potyviruses from tulip and lily which cause flower-breaking. J Gen Virol 74: 881–887
Devereux J, Haeberli P, Smithies O (1984) A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res 12: 387–395
Dinant S, Lot H, Albouy J, Kuziak C, Meyer M, Astier-Manifacier S (1991) Nucleotide sequence of the 3' terminal region of lettuce mosaic potyvirus RNA shows a Gln/Val dipeptide at the cleavage site between the polymerase and the coat protein. Arch Virol 116: 235–252
Domier LL, Franklin KM, Shahabuddin M, Hellmann GM, Overmeyer JH, Hiremath ST, Siaw MFE, Lomonosoff GP, Shaw JG, Rhoads RE (1986) The nucleotide sequence of tobacco vein mottling virus RNA. Nucleic Acids Res 14: 5417–5430
Domier LL, Shaw JG, Rhoads RE (1987) Potyviral proteins share amino acid sequence homology with picorn-, como-, and caulimovirus proteins. Virology 158: 20–27
Dougherty WG, Carrington JC (1988) Expression and function of potyviral gene products. Annu Rev Phytopathol 26: 123–143
Eagles RM, Gardner RC, Forster RLS (1990) Nucleotide sequence of the tamarillo mosaic virus coat protein gene. Nucleic Acids Res 18: 7166
Fang H, Nee H, Chou T (1985) Comparative ability of seventeen aphid species to transmit tobacco vein-banding mosaic virus. Bull Taiwan Tob Res Inst 22: 41–46
Felsenstien J (1988) Phylogenies from molecular sequences: Inference and reliability. Annu Rev Genet 22: 521–565
Frenkel MJ, Jilka JM, McKern NM, Strike PM, Clark JM, Shukla DD, Ward CW (1991) Unexpected sequence diversity in the amino-terminal ends of the coat proteins of strains of sugarcane mosaic virus. J Gen Virol 72: 237–242
Frenkel MJ, Ward CW, Shukla DD (1989) The use of 3' non-coding nucleotide sequences in the taxonomy of potyviruses: Application to watermelon mosaic virus 2 and soybean mosaic virus-N. J Gen Virol 70: 2775–2783
Gough KH, Azad AA, Hanna PJ, Shukla DD (1987) Nucleotide sequence of the capsid and nuclear inclusion protein genes from the johnson grass strain of sugarcane mosaic virus RNA. J Gen Virol 68: 297–304
Hammond J, Hammond RW (1989) Molecular cloning, sequencing and expression inEscherichia coli of the bean yellow mosaic virus coat protein gene. J Gen Virol 70: 1961–1974
Hammond J, Lawson RH (1988) An improved purification procedure for preparing potyviruses and cytoplasmic inclusions from the same tissue. J Virol Methods 20: 203–217
Hunt AG, Chu NM, Odell JT, Nagy F, Chua N-H (1987) Plant cells do not properly recognize animal gene polyadenylation signals. Plant Mol Biol 8: 23–35
Jayram C, Hill JH, Miller WA (1992) Complete nucleotide sequences of two soybean mosaic virus strains differentiated by response of soybean containing theRsv resistance gene. J Gen Virol 73: 2067–2077
Johansen E, Rasmussen OF, Heide M, Borkhardt B (1991) The complete nucleotide sequence of pea seed-borne mosaic virus RNA. J Gen Virol 72: 2624–2632
Kashiwazaki S, Hayano Y, Minobe Y, Omura T, Hibino H, Tsuchizaki T (1989) Nucleotide sequence of the capsid protein gene of barley yellow mosaic virus. J Gen Virol 70: 3015–3023
Khan JA, Lohuis D, Goldbach R, Dijkstra J (1993) Sequence data to settle the taxonomic position of bean common mosaic virus and blackeye cowpea mosaic virus isolates. J Gen Virol 74: 2243–2249
Kimura M (1980) A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16: 111–120
Laín S, Reichmann JL, Garcia JA (1989) The complete nucleotide sequence of plum pox potyvirus RNA. Virus Res 13: 157–172
Niblett CL, Zagula KR, Clavert LA, Kendall TL, Stark DM, Smith CE, Beachy RN, Lommel SA (1991) cDNA cloning and nucleotide sequence of the wheat streak mosaic virus capsid protein gene. J Gen Virol 72: 499–504
Nicolas O, Laliberté JF (1992) The complete nucleotide sequence of turnip mosaic potyvirus RNA. J Gen Virol 73: 2785–2793
Pappu S, Brand R, Pappu H, Rybicki E, Gough K, Frenkel M, Niblett C (1993) A polymerase chain reaction method adapted for selective amplification and cloning of 3' sequences of potyviral genomes: Application to dasheen mosaic virus. J Virol Methods 41: 9–20
Quemada H, L'Hostis B, Gonsalves D, Reardon IM, Heinrikson R, Hiebert EL, Sieu L, Slightom JL (1990) The nucleotide sequences of the 3'-terminal regions of papaya ringspot virus strains W and P. J Gen Virol 71: 203–210
Quemada H, Sieu LC, Siemieniak DR, Gonsalves D, Slightom JL (1990) Watermelon mosaic virus II and zucchini yellow mosaic virus: cloning of 3'-terminal regions, nucleotide sequences, and phylogenetic comparisons. J Gen Virol 71: 1451–1460
Ravelonandro M, Varveri C, Delbos R, Dunez J (1988) Nucleotide sequence of the capsid protein gene of plum pox potyvirus. J Gen Virol 69: 1509–1516
Reddick BB, Barnett OW (1983) A comparison of three potyviruses by direct hybridization analysis. Phytopathology 73: 1506–1510
Reddick BB, Collins-Shepard MH, Christie RG, Gooding GV (1992) A new virus disease in North America caused by tobacco vein-banding mosaic virus. Plant Dis 76: 856–859
Robaglia C, Durand-Tardif M, Tronchet M, Boudazin G, Astier-Manifacier S, Casse-Delbart F (1989) Nucleotide sequence of potato virus Y (N strain) genomic RNA. J Gen Virol 70: 935–947
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4: 406–425
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning. A laboratory manual. 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74: 5463–5467
Shukla DD, Frenkel MJ, Ward CW (1991) Structure and function of the potyvirus genome with special reference to the coat protein coding region. Can J Plant Pathol 13: 189–191
Shukla DD, Ward CW (1988) Amino acid sequence homology of coat proteins as a basis for identification and classification of the potyvirus group. J Gen Virol 69: 2703–2710
Swofford DL (1993) PAUP: Phylogenetic Analysis Using Parsimony, Version 3.1. Computer program distributed by the Illinois Natural History Survey, Champaign, IL
Tracy SL, Frenkel MJ, Gough KH, Hanna PJ, Shukla DD (1992) Bean yellow mosaic, clover yellow vein, and pea mosaic are distinct potyviruses: evidence from coat protein gene sequences and molecular hybridization involving the 3′ non-coding regions. Arch Virol 122: 249–261
Vance VB, Moore D, Turpen TH, Bracker A, Hollowell VC (1992) The complete nucleotide sequence of pepper mottle virus genomic RNA: potyviruses. Virology 191: 19–30
Vetten HJ, Lesemann D-E, Maiss E (1992) Serotype A and B strains of bean common mosaic virus are two distinct potyviruses. In: Barnett OW (ed) Potyvirus taxonomy. Springer, Wien New York, pp 415–431 (Arch Virol [Suppl] 5)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Habera, L.F., Berger, P.H. & Reddick, B.B. Molecular evidence from 3′-terminus sequence analysis that tobacco vein-banding mosaic virus is a distinct member of the potyvirus group. Archives of Virology 138, 27–38 (1994). https://doi.org/10.1007/BF01310036
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01310036