Summary
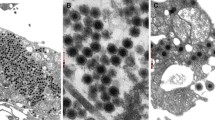
A virus isolated from the internal organs of a moribund corn snake (Elaphe guttata) replicated in reptilian cell cultures (IgH-2, TH-1 cells) between 10 and 30°C. Highest infectivity titers of 105.5 TCID50/ml were obtained in IgH-2 cells at 25°C. Infected IgH-2 cells showed the development of three morphologically different intranuclear inclusion bodies. During viral assembly the particles formed typical crystalline aggregates in the nucleus. About 64 h after infection progressive desintegration of the nuclear membrane was evident and virus particles were released into the cytoplasm. Different fish cell lines (CLC, CHSE-214, BF-2, PG, RTG-2) were not capable of propagating the virus. The DNA containing agent proved to be stable at pH 3, more or less at pH 12 and to treatment with chloroform, but it was rapidly inactivated at 56°C. Electron microscopy revealed nonenveloped icosahedral particles with a diameter of 65–70 nm.
Similar content being viewed by others
References
Adair BM, Curran WL, McFerran JB (1979) Ultrastructural studies of the replication of fowl adenoviruses in primary cell cultures. Avian Pathol 8: 133–144
Ahne W (1979) Fish cell cultures: a fibroblastic line (PG-cells) from ovaries of juvenile pike (Esox lucius). In Vitro 11: 65–69
Ahne W (1991) Viral infections in reptiles. In: Proceedings of the 4th International Colloquium on Pathology and Medicine of Reptiles and Amphibians. Deutsche Veterinärmedizinische Gesellschaft, Giessen, pp 1–12
Ahne W, Schlotfeldt HJ, Thomsen I (1989) Fish viruses: isolation of an icosahedral cytoplasmic deoxyribovirus from sheatfish (Silurus glanis). J Vet Med B 36: 333–336
Barski G, Cornefert F (1958) Aspects distinctifs des lésions cellulaires causées in vitro par différents types d'Adénovirus. Ann Inst Past 94: 724–731
Boyer GS, Denny FW Jr, Miller I, Ginsberg HS (1960) Correlation of production of infectious virus with sequential stages of cytological alteration in He-La cells infected with adenoviruses types 5 and 7. J Exp Med 112: 865–882
Clark HF, Karzon DT (1967) Terrapene heart (TH-1), a continuous cell line from the heart of the box turtleTerrapene carolina. Exp Cell Res 48: 263–268
Clark HF, Cohen MM, Karzon DT (1970) Characterization of reptilian cell lines established at incubation temperatures of 23 to 36°C. Proc Soc Exp Biol Med 133: 1039–1047
Clark HF, Michalski F, Tweedell KS, Yohn D, Zeigel RF (1973) An adenovirus, FAV-1, isolated from the kidney of a frog (Rana pipiens). Virology 51: 392–400
Dales S (1962) An electron microscope study of the early association between two mammalian viruses and their hosts. J Cell Biol 13: 303–322
Faisal M, Ahne W (1990) A cell line (CLC) of adherent peripheral blood mononuclear cells of a normal common carp (Cyprinus carpio). Dev Comp Immunol 14: 255–260
Russell WC (1991)Adenoviridae. In: Francki RIB, Fauquet CM, Knudson DL, Brown F (eds) Classification and nomenclature of viruses. Arch Virol [Suppl] 2: 140–144
Freyer JL, Yusha A, Pilcher AS (1965) The in vitro cultivation of tissue and cells of Pacific salmon and steelhead trout. Ann NY Acad Sci 126: 566–571
Gravell M, Malsberger RG (1965) A permanent cell line from the fathead minnow (Pimephales promelas). Ann NY Acad Sci 126: 555–565
Harford CG, Hamlin A, Parker E, van Ravenswaay T (1956) Electron microscopy of HeLa cells infected with adenoviruses. J Exp Med 104: 443–465
Heldstab A, Bestetti G (1984) Virus associated gastrointestinal disease in snakes. J Zoo Anim Med 15: 118–128
Horwitz MS (1985) Adenoviruses and their replication. In: Fields BN, Chanock RM, Knipe DM, Melnick JL, Roizmann B, Shope RE (eds) Fields virology. Raven, New York, pp 433–476
Jacobson ER (1986) Viruses and viral associated diseases of reptiles. In: Bels VL, Van den Sande AP (eds) Maintenance and reproduction of reptiles in captivity, vol II. Acta Zool Pathol Antverp 79: 73–90
Jacobson ER, Gardiner CH (1990) Adeno-like virus in esophageal and tracheal mucosa of a Jackson's chameleon (Chamaeleo jacksoni). Vet Pathol 27: 210–212
Jacobson ER, Gardiner CH, Foggin CM (1984) Adenovirus-like infection in two Nile crocodiles. J Am Vet Med Assoc 185: 1421–1422
Jacobson ER, Gaskin JM, Gardiner CH (1985) Adenovirus-like infection in aBoa constrictor. J Am Vet Med Assoc 187: 1226–1227
Kaerber G (1931) Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Arch Exp Pathol Pharmakol 162: 480–483
Lapis K (1965) Electronmicroscopic examination of KB cell cultures infected with adenovirus type 12. Acta Microbiol Acad Sci Hung 12: 241–259
Liao SK, Weber J (1969) Cytopathogenicity and replication of human adenovirus type 6 in cultured bovine cells. Can J Microbiol 15: 847–850
Maeda M, Okaniwa A, Kawamura H (1967) Morphological studies on intranuclear inclusion bodies in chicken kidney cell culture infected with avian adenovirus. Natl Inst Anim Health 7: 164–177
Martinez-Palomo A, Le Buis J, Bernhard W (1967) Electron microscopy of adenovirus 12 replication. 1. Fine structural changes in the nucleus of infected KB cells. J Virol 1: 817–829
Shahrabadi MS, Marusyk RG, Crawford TB (1977) Electronmicroscopic study of the development of an equine adenovirus in cultured fetal equine kidney cells. Can J Microbiol 23: 497–509
Shortridge KF (1989) Viruses of reptiles. In: Ahne W, Kurstak E (eds) Viruses of lower vertebrates. Springer, Berlin Heidelberg New York Tokyo, pp 89–104
Wittekind D (1979) On the nature of Romanowsky dyes and the Romanowsky-Giemsa effect. Clin Lab Haematol 1: 247–262
Wolf K, Quimby MC (1962) Established eurythermic line of fish cells in vitro. Science 125: 1065
Wong WF, Tweedel KS (1974) Two viruses from the Lucké tumour isolated in frog pronephric cell line. Soc Exp Biol Med Proc 145: 1201–1206
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Juhasz, A., Ahne, W. Physicochemical properties and cytopathogenicity of an adenovirus-like agent isolated from corn snake (Elaphe guttata). Archives of Virology 130, 429–439 (1993). https://doi.org/10.1007/BF01309671
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01309671