Summary
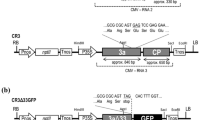
The coat protein (CP) of strain SC of sugarcane mosaic virus (SCMV-SC) was expressed transiently in sugarcane protoplasts after electroporation with one of two plasmids encoding the CP gene. The CP gene was fused with either the cauliflower mosaic virus 35S promoter or the synthetic monocotyledon promoter “Emu”. The coat protein gene was also inducibly expressed inEscherichia coli when fused to the trc promoter. The protein expressed in both systems had the same electrophoretic mobility and antigenic specificity as purified SCMV-SC coat protein. Transient expression of the 35S-CP gene in protoplasts could only be demonstrated in Western blots developed with the chemiluminescence enzyme substrate luminol.
Similar content being viewed by others
References
Amersham (1990) ECL detection of nucleic acid and proteins with light. Amersham International, England
Dougherty WG, Carrington JC (1988) Expression and function of potyviral gene products. Annu Rev Phytopathol 26: 123–143
Dunn SD (1986) Effects of the modification of transfer buffer composition and the renaturation of proteins in gels on the recognition of proteins on western blots by monoclonal antibodies. Anal Biochem 157: 144–153
Frenkel MJ, Jilka JM, McKern NM, Strike PM, Clark JM Jr, Shukla DD, Ward CW (1991) Unexpected sequence diversity in the amino-terminal ends of the coat proteins of strains of sugarcane mosaic virus. J Gen Virol 72: 237–242
Fromm ME, Taylor LP, Walbot V (1985) Expression of genes transferred into monocot and dicot plant cells by electroporation. Proc Natl Acad Sci USA 82: 151–158
Hauptmann RM, Ozias-Akins P, Vasil V, Tabaeizadeh Z, Rogers SG, Horsch RB, Vasil IK, Fraley RT (1987) Transient expression of electroporated DNA in monocotyledonous and dicotyledonous species. Plant Cell Rep 6: 265–270
Kozak M (1984) Point mutations close to the AUG initiator codon affect the efficiency of translation of rat preproinsulin in vivo. Nature 308: 241–246
Last DI, Brettell RIS, Chamberlain DA, Chaudhury AM, Larkin PJ, Marsh EL, Peacock WJ, Dennis ES (1990) pEmu: an improved promoter for gene expression in cereal cells. Theor Appl Genet 81: 581–588
Lutcke HA, Chow KC, Mickel FS, Moss KA, Kern HF, Schelle GA (1987) Selection of AUG initiation codons differs in plants and animals. EMBO J 6: 43–48
Powell Abel P, Nelson RS, Barum D, Hoffman N, Rogers SG, Fraley RT, Beachy RN (1986) Delay of disease development in transgenic plants that express the tobacco mosaic virus coat protein gene. Science 232: 738–743
Rathus C, Birch RG (1992) Optimisation of conditions for electroporation and transient expression of foreign genes in sugarcane protoplasts. Plant Sci 81: 65–74
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning. A laboratory manual, 2nd edn. Cold Spring Harbour Laboratory Press, New York
Shukla DD, Gough KH (1984) Serological relationships among four Australian strains of sugarcane mosaic virus as determined by immune electron microscopy. Plant Dis 68: 204–206
Shukla DD, Ward CW (1989) Structure of potyvirus coat proteins and its application in the taxonomy of the potyvirus group. Adv Virus Res 36: 273–314
Shukla DD, Frenkel MJ, Ward CW (1991) Structure and function of potyvirus genome with special reference to the coat protein coding region. Can J Plant Pathol 13: 178–191
Srinivasan C, Vasil IK (1986) Plant regeneration from protoplasts of sugarcane (Saccharum officinarum L.). J Plant Physiol 126: 41–48
Taylor PWJ, Ko H-L, Adkins SW, Rathus C, Birch RG (1992) Establishment of embryogenic callus and high protoplast yielding suspension cultures of sugarcane (Saccharum spp hybrids). Plant Cell Tissue Organ Cult 28: 69–78
Teakle DS, Grylls NE (1973) Four strains of sugarcane mosaic virus infecting cereals and other grasses in Australia. Austral J Agric Res 24: 465–477
Teakle DS, Shukla DD, Ford RE (1990) Sugarcane mosaic virus (revised). AAB Descriptions of Plant Viruses, no 342
Tomes DT, Ross M, Higgens R, Rao AG, Staebell M, Howard J (1990) Direct DNA transfer into intact plant cells and recovery of transgenic plants via microprojectile bombardment. In: Gelvin SB, Schilperoort RA, Verma DPS (eds) Plant molecular biology manual A 13. Kluwer, Dordrecht, pp 1–22
Tumer NE, O'Connell KM, Nelson RS, Sanders PR, Beachy RN, Fraley RT, Shah DM (1987) Expression of alfalfa mosaic virus coat protein gene confers cross-protection in transgenic tobacco and tomato plants. EMBO J 6: 1181–1188
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Smith, G.R., Ford, R., Frenkel, M.J. et al. Transient expression of the coat protein of sugarcane mosaic virus in sugarcane protoplasts and expression inEscherichia coli . Archives of Virology 125, 15–23 (1992). https://doi.org/10.1007/BF01309625
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01309625