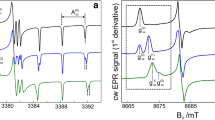
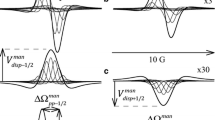
Abstract
Methods of EPR spectroscopy and GLC were used to determine the magnetic resonance and thermodynamic characters of the formation of paramagnetic complexes consisting of a free π radical (diphenyldicumynitroxyl, triphenylverdazyl, indophenoxyl) and substituted benzenes. It was established that in such systems, depending on the donor-acceptor and polar properties of the diamagnetic molecules, there form paramagnetic complexes of two types: donor-acceptor complexes in which the free radical acts as a β-electron acceptor and both intramolecular and intermolecular redistribution of spin density is accomplished, and dipole-dipole complex in which electrostatic interaction determines mutual orientation of molecules in the complex and intramolecular distribution of electron and spin densities. Spectral and thermodynamic criteria of the formation of donor-acceptor and dipole-dipole paramagnetic free radical-diamagnetic molecule complexes are formulated.
Similar content being viewed by others
Literature cited
A. L. Buchachenko and A. M. Vasserman, Stable Radicals [in Russian], Khimiya, Moscow (1973).
A. L. Buchachenko, Complexes of Radicals and Molecular Oxygen with Organic Molecules [in Russian], Nauka, Moscow (1984).
A. Yu. Karmilov and N. A. Sysoeva, “Study of the interactions of nitroxyl radical with polar molecules by the EPR method,” Zh. Fiz. Khim.,55, No. 4, 950–954 (1981).
V. S. Kuts, N. F. Radchenko, and V. D. Pokhodenko, “On the electronic structure of free nitroxyl radical-aromatic molecule π complexes,” Teor. Eksp. Khim.,16, No. 5, 626–635 (1980).
V. S. Kuts, N. F. Radchenko, and V. D. Pokhodenko, “On the effect of the nature of the interacting molecular orbitals on magnetic resonance parameters of free radicals in their complexes with aromatic molecules,” Teor. Eksp. Khim.,17, No. 2, 196–202 (1981).
V. S. Kuts, T. S. Slipenyuk, and E. P. Platonova, “Effect of electronic structure of free radical on complex formation with diamagnetic molecule,” Teor. Eksp. Khim.,13, No. 3, 357–369 (1977).
V. S. Kuts and T. S. Slipenyuk, “Radiospectroscopic study of intermolecular interactions in paramagnetic systems,” Spectroscopy of Molecules and Crystals [in Russian], Part 2, Nauk. Dumka, Kiev (1980), pp. 182–194.
N. F. Radchenko, G. V. Filonenko, and V. S. Kuts, “Investigation of the effect of the structure of free nitroxyl radical-aromatic ligand complexes on their thermodynamic characteristics,” Teor. Eksp. Khim.,17, No. 6, 774–779 (1981).
L. Butt, G. M. Burnett, and J. Cameron, “Determination of stability constants by gasliquid chromatography for the complexing of aromatic solvents and monomers with a nitroxide radicals,” J. Chem. Soc. Chem. Commun., No. 1, 29–80 (1971).
G. M. Burnett, G. G. Cameron, and J. Cameron, “Stability constants of some complexes of t-butyl-mesityl nitroxide,” J. Chem. Soc. Faraday Trans. I., NO. 5, 864–870 (1973).
A. I. Chernyavskii and V. A. Poluektov, “Method of chromatographic determination of dipole moments and energy of orientation interaction,” Zh. Fiz. Khim.,53, No. 8, 2052–2055 (1979).
O. M. Polumbrik, The Chemistry of Verdazyl Radicals [in Russian], Nauk. Dumka, Kiev (1984).
V. D. Pokhodenko, A. A. Beloded, and V. G. Koshechko, Oxidation-Reduction Reactions of Free Radicals [in Russian], Nauk. Dumka, Kiev (1973).
V. D. Pokhodenko, E. P. Platonova, N. F. Radchenko, and V. S. Kuts, “On the nature of boundary molecular orbitals that participate in electrode redox reactions of nitroxyl radicals,” Elektrokhimiya,20, No. 11, 1451–1456 (1984).
E. P. Platonova, V. D. Pokhodenko, and E. N. Negoda, “Electrochemical oxidation of nitroxyl radicals,” Elektrokhimiya,13, No. 3, 391–393 (1977).
E. G. Rozantsev and V. D. Sholle, Organic Chemistry of Free Radicals [in Russian], Khimiya, Moscow (1978).
V. D. Pokhodenko, V. S. Kuts, and A. M. Golubenkova, “Thermodynamic and kinetic characteristics of reaction with transfer of one electron in systems with N-alkylanilinestetracyanoethylene CTC,” Teor. Eksp. Khim.,23, No. 6, 692–699 (1987).
A. Gordon and R. Ford, The Chemist's Companion [Russian translation], Mir, Moscow (1976).
Author information
Authors and Affiliations
Additional information
Translated from Teoreticheskaya i Éksperimental'naya Khimiya, Vol. 25, No. 3, pp. 283–289, May–June, 1989.
Rights and permissions
About this article
Cite this article
Kuts, V.S., Radchenko, N.F. & Filonenko, G.V. Relationship of magnetic resonance, thermodynamic, and structural characteristics of π complexes of free radicals with aromatic molecules. Theor Exp Chem 25, 260–265 (1989). https://doi.org/10.1007/BF01299001
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01299001