Abstract
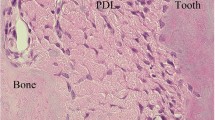
Fibroblasts isolated from human periodontal ligament (PDL) were cultured under a medium supplemented with ascorbic acid, β-glycerophosphate and dexamethasone. The cultures were assessed for their ability to elaborate a mineralized matrix.
Cell cultures stained positive when analyzed for alkaline phosphatase activity throughout the culture period. After about 3 weeks in culture, the cells produced a calcified matrix. Light microscopy showed formation of clusters of different shapes and sizes. Von Kossa staining revealed mineral deposits as amorphous brown-black precipitates. Transmission electron microscopy showed cells in multilayers and mineralized formations in close association with a dense network of collagen fibers. Scanning electron microscopy revealed smooth formations rising over the cultures with an abundant fiber matrix.
We conclude that human PDL fibroblasts can be induced to form a mineralized matrix which shares features with bone mineralized matrix but most likely represents a more immature type ofin vitro mineralization. Moreover, the present study further supports the osteoblastic potential of these cells.
Zusammenfassung
Fibroblasten des humanen Parodontalligaments (PDL) wurden in einem Medium, das mit Vitamin C, β-Glycerophosphat und Dexamethason angereichert war, kultiviert. Diese Kulturen wurden auf ihre Fähigkeit zur Bildung einer mineralisierten Matrix hin untersucht.
In diesen Zellkulturen verlief der Nachweis der alkalischen Phosphatase während des gesamten Kulturzeitraums positiv. Nach einem Kulturzeitraum von etwa drei Wochen produzierten die Zellen eine kalzifizierte Matrix. Lichtmikroskopisch war die Bildung von Zellklustern (Zellaggregaten) unterschiedlicher Gestalt und Größe sichtbar. Die Von-Kossa-Färbung ließ mineralisierte Ablagerungen in Form amorpher braun-schwarzer Präzipitate erkennen. Die Transmissionselektronenmikroskopie zeigte eine mehrschichtige Anordnung der Zellen und bestätigte mineralisierte Ablagerungen, die in enger Verbindung mit einem dichten Netzwerk aus Kollagenfasern standen. Die rasterelektronenmikroskopische Untersuchung verdeutlichte, daß die Kulturen mit Ablagerungen überschichtet waren, die auf die Gegenwart einer faserartigen Matrix hindeuteten.
Die Befunde lassen den Schluß zu, daß in humanen PDL-Fibroblasten die Bildung einer mineralisierten Matrix induziert werden kann, die Charakteristika der mineralisierten Matrix von Knochen aufweist, aber weitestgehend eine eher unreife Form derIn-vitro-Mineralisation darstellt. Darüber hinaus bestätigt die vorliegende Untersuchung das Potential dieser Zellen, Charakteristika von Osteoblasten zu realisieren.
Similar content being viewed by others
References
Alliot-Licht B, De Lange GI, Gregoire M. Effects of hydroxyapatite particles on periodontal fibroblast-like cell behavior. J Periodontol 1997; 68:158–65.
Arceo N, Sauk JJ, Moehring J, et al. Human periodontal cells initiate mineral-like nodules in vitro. J Periodontal 1991; 62:499–503.
Aubin JE, Heersche JNM, Merrilees MJ, et al. Isolation of bone cell clones with difference in growth, hormone responses, and extracellular matrix production. J Cell Biol 1982; 92:452–61.
Basdra EK, Kohl A, Komposch G. Mechanical stretching of periodontal ligament fibroblasts — a study on cytoskeletal involvement. J Orofac Orthop 1996; 57:24–30.
Basdra EK, Komposch G, Huber LA, et al. Mechanically stretched periodontal ligament fibroblasts: Identifying elements of the mechanotransduction cascade. In: Davidovitch Z, ed. Biological mechanisms of tooth eruption and replacement by implants. Birmingham Alabama: EBSCO Media, 1996:41–9.
Basdra EK, Komposch G. Osteoblast-like properties of human periodontal ligament cells: an in vitro analysis. Eur J Orthod 1997; 19:615–21.
Basdra EK, Papavassiliou AG, Huber LA. Rab and rho GTPases are involved in specific response of periodontal ligament fibroblasts to mechanical stretching. Biochim Biophys Acta 1995;1268:209–13.
Beertsen W, Everts V. Formation of acellular root cementum in relation to dental and non-dental hard tissues in the rat. J Dent Res 1990;1669–73.
Bellows CG, JE Aubin, JNM Heersche, et al. Minteralized bone nodules formed in vitro from enzymatically released rat calvaria cell populations. Calcif Tissue Int 1986; 38:143–54.
Bhargava U, Bar-Lev M, Bellows CG, et al. Ultrastructural analysis of bone nodules formed in vitro by isolated rat calvaria cells. Bone 1988;9:155–63.
Cho MJog, Matsuda N, Lin WL, et al. In vitro formation of mineralized nodules by periodontal ligament cells from the rat. Calcif Tissue Int 1992;50:459–67.
Escarot-Charrier B, Shepard N, Charette G, et al. Mineralization in osteoblast cultures: A light and electron microscopic study. Bone 1988;9:147–54.
Gerstenfeld LC, Chipman SD, Glowaski J, et al. Expression of differentiated function by mineralizing cultures of chicken osteoblasts. Dev Biol 1987;122:49–60.
Gerstenfeld LC, Chipman SD, Kelly CM, et al. Collagen expression, ultrastructural assembly and mineralization in cultures of chicken embryo osteoblasts. J Cell Biol 1988;106:979–89.
Lin WL, McCulloch CA, Cho MI. Differentiation of periodontal ligament fibroblasts into ostoblasts during socket healing after tooth extraction in the rat. Anat Rec 1994;240:492–506.
Luben RA, Wong GL, Cohn DV. Biochemical characterization with parathormone and calcitonin of isolated cells: provisional identification of osteoclasts and osteoblasts. Endocrinology 1976;99:526–34.
McCulloch CAG, AH Melcher. Continuous labelling of the periodontal ligament of mice. J Periodont Res 1982;18:231–41.
McCulloch CAG, Melcher AH. Cell density and cell generation in the periodontal ligament of mice. Am J Anat 1983;167;43–58.
Nefussi JR, Boy-Lefevre ML, Boulekbache H, et al. Mineralization in vitro of matrix formed by osteoblasts isolated by collagenase digestion. Differentiation 1985;29:160–8.
Nojiima N, Kobayashi M, Shionone M, et al. Fibroblastic cells derived from bovine periodontal ligaments have the phenotypes of osteoblasts. J Period Res 1990;25:179–85.
Owen ME. Lineage of osteogenic cells and their relationship to the stromal system: Bone Mineral Res 1985; 1–25.
Piche JE, Carnes EDL, Graves DT. Initial characterization of cells derived from human periodontia. J Dent Res 1989;68:761–7.
Roberts WE, Morey ER. Proliferation and differentiation sequence of osteoblast histogenesis under physiologic conditions in rat periodontal ligament. Am J Anat 1985;174:105–18.
Roberts WE, Wood HB, Chambers DW, et al. Vascularly oriented differentiation gradient of osteoblast precursor cells in rat periodontal ligament: Implications for osteoblast histogenesis and periodontal bone loss. J Periodont Res 1987;22:461–7.
Schroeder HE. Oral structural biology. Stuttgart: Thieme, 1991.
Somerman MJ, Archer SY, Imm GR, et al. A comparative study of human periodontal ligament cells and gingival fibroblasts in vitro. J Dent Res 1988;67:66–70.
Somerman MJ, Young MA, Foster RA, et al. Characteristics of human periodontal ligament cells in vitro. Arch Oral Biol 1990;35:241–7.
Sudo HA, Kodama HA, Amagai Y. In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J Cell Biol 1983;96:191–8.
Ten Cate AR. Oral histology, development, structure, and function. 4th edn. St Louis: Mosby, 1994:276–312.
Tenebaum HC, Heersche JNM. Differentiation of osteoblasts and formation of mineralized bone in vitro. Calcif Tissue Int 1982;34:76–9.
Urist MR, Delange RJ, Finerman GAM. Bone cell differentiation and growth factors. Science 1983;220:680–6.
Weinger JM, Holtrop ME. An ultrastructural study of bone cells: The occurrence of microtubules, microfilaments and tight junctions. Calcif Tissue Res 1973;14:15–29.
Yamaguchi M, Shimizu N, Shibata Y, et al. Effects of different magnitudes of tension-force on alkaline phosphotase activity in periodontal ligament cells. J Dent Res 1996; 75:889–94.
Author information
Authors and Affiliations
Additional information
Dedicated to Prof. Dr. Emil Witt on the Occasion of his 65th Birthday.
Rights and permissions
About this article
Cite this article
Basdra, E.K., Komposch, G. Transmission and scanning electron microscopic analysis of mineralized loci formed by human periodontal ligament cellsin vitro . J Orofac Orthop/Fortschr Kieferorthop 60, 77–86 (1999). https://doi.org/10.1007/BF01298958
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01298958