Conclusions
The destruction of tin-oxide ceramic by glass takes place rapidly, despite the absence of marked penetration of molten glass into the pores of the material, i.e., the low impregnation is not an essential condition for high corrosion resistance.
The corrosion is linked with the change in the structure of the material, expressed by the increase in its dispersion and the development of a large number of fine, weakly interbonded grains with subsequent penetration between them of glass, bloating, and breakdown into separate fragments.
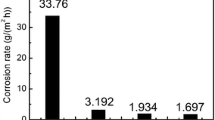
The least corrosion by lead glass is exhibited by tin oxide ceramic with clearly expressed crystals bonded by dense intergrain contacts, regardless of the total porosity.
The most intense corrosion is typical of a material with badly formed intergrain faces, and a high open porosity. Even prolonged service at high temperatures in contact with glass does not lead to marked growth of the grains and their regular delimitation.
The most resistant to corrosion turned out to be a material with a well-formed granular structure, with a pore volume of about 9.1 mm3/g, and pores measuring 10–20 μm. In service it is completely impregnated with glass.
Similar content being viewed by others
Literature cited
S. I. Matyusha, I. E. Bekker, T. A. Zhuravina, et al., Abstract information All Union Inst. for Scientific, Technical Information and Economics of the Building Materials Industry, Ser. Glass Industry, Min-vo Prom-ti Stroit, Mat-lov SSSR (1974), No. 7.
K. A. Konstanyan, A. F. Melik-Akhnazarov, et al., Steklo Keram., No. 4, 6–8 (1976).
Ya. Stanek, Electric Melting of Glass [Russian translation], Legkaya Industriya Moscow, (1979), p. 248.
G. I. Astanina, V. L. Balkevich, et al., Steklo Keram., No. 2, 28–30 (1983).
Ya. E. Geguzin, Physics of Sintering [in Russian], Nauka, Moscow (1984), p. 312.
A. Adamson, Physical Chemistry of Surfaces [Russian translation], Mir, Moscow (1979), p. 568.
F. S. Kaplan and T. L. Kalyada, Ogneupory, No. 12, 49–54 (1982).
G. A. Aksel'rud and M. A. Al'tshuler, Introduction to Capillary Chemical Technology [in Russian], Khimiya, Moscow (1983), p. 263.
P. A. Rebinder and E. D. Shchukin, Surface Phenomena in Disperse Systems. Physicochemical Mechanics [in Russian], Nauka, Moscow (1979), pp. 203–268.
N. V. Pertsov, Physicochemical Mechanics and the Liophyllic Nature of Dispersed Systems. Collection of Papers [in Russian], No. 18, Naukova Dumka, Kiev (1986), pp. 17–18.
A. A. Verlotskii, I. P. Rublevskii, V. P. Frolov, et al., Steklo Keram., No. 2, 5–11 (1987).
Author information
Authors and Affiliations
Additional information
Translated from Ogneupory, No. 2, pp. 24–29, February, 1989.
Rights and permissions
About this article
Cite this article
Mel'nikova, I.G., Kaplan, F.S. & Fedorova, V.V. Mechanism of corrosion of tin-oxide electrodes. Refractories 30, 91–96 (1989). https://doi.org/10.1007/BF01292548
Issue Date:
DOI: https://doi.org/10.1007/BF01292548