Summary
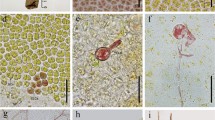
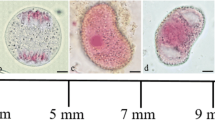
The antheridium ofChara vulgaris L. is connected by plasmodesmata with the thallusvia a basal cell. Prior to the initiation of spermatozoid differentiation these plasmodesmata are spontaneously broken, resulting in symplasmic isolation of the antheridium.
Premature plasmolytically evoked symplasmic isolation of the antheridium leads to a 2–4 fold reduction in the length of antheridial filaments and the elimination of 1–2 cell cycles from the first stage of spermatogenesis.
Autoradiographic and cytophotometric studies have shown that, as a result of induced symplasmic isolation of the antheridium, endomitotic DNA synthesis was blocked both in the young manubria (after 24 hours) and in the capitular cells (after 48 hours). In the antheridial filaments DNA synthesis was inhibited together with either elimination of divisions and induction of spermatid differentiation or developmental block. We propose that breakage of plasmodesmata connecting the antheridium with the thallus is a signal which releases, in all antheridia, mechanisms that (i) block endomitotic DNA synthesis in the manubria, (ii) restrict the growth rate and the divisions of antheridial filament cells, and (iii) induce spermiogenesis in these antheridia in which the manubria attained the sufficient level of polyploidy.
Similar content being viewed by others
References
Allaway WG, Setterfield G (1972) Ultrastructural observations on guard cells ofVicia faba andAllium porrum. Can J Bot 50: 1405–1413
Button J, Kochba J, Bornman CH (1974) Fine structure of and embryoid development from embryogenic ovular callus of “Shamouti” orangeCitrus sinensis Osb. J exp Bot 25: 446–457
Carr DJ (1976) Plasmodesmata in growth and development. In:Gunning BES, Robards AW (eds) Intercellular communication in plants: Studies on plasmodesmata. Springer, Berlin Heidelberg New York, pp 243–289
Forsberg C (1965) Nutritional studies ofChara in axenic cultures. Physiol Plant 18: 275–289
Goodwin PB, Lyndon RF (1983) Synchronisation of cell division during transition to flowering inSilene apices not due to increased symplast permeability. Protoplasma 116: 219–222
Gunning BES (1978) Age-related and origin-related control of the numbers of plasmodesmata in cell walls of developingAzolla roots. Planta 143: 181–190
Juniper BE (1977) Some speculations on the possible roles of the plasmodesmata in the control of differentiation. J Theor Biol 66: 583–592
Kwiatkowska M, Maszewski J (1976) Plasmodesmata between synchronously and asynchronously developing cells of the anheridial filaments ofChara vulgaris L. Protoplasma 87: 317–327
Kwiatkowska M, Maszewski J (1985 a) Changes in ultrastructure of plasmodesmata during spermatogenesis inChara vulgaris L. Planta 166: 46–50
— — (1985 b) Nucleolar size and the activity of rRNA transport in the course of morphogenetic reduction of cell dimensions. Folia Histochem Cytobiol 23: 135–144
— — (1986) Changes in the occurrence and ultrastructure of plasmodesmata in antheridia ofChara vulgaris L. during different stages of spermatogenesis. Protoplasma 132: 179–188
— — (1987 a) Cell size and DNA level as related to the initiation of spermatozoids differentiation in antheridial filaments ofChara vulgaris L. Folia Histochem Cytobiol 24: 79–90
— — (1987 b) Ultrastructural and autoradiographic studies of nongenerative cells in the antheridium ofChara vulgaris L. I. Manubria. Folia Histochem Cytobiol 25: 215–223
— — (1987 c) Ultrastructural and autoradiographic studies of nongenerative cells in the antheridium ofChara vulgaris L. II. Capitular cells. Folia Histochem Cytobiol 25: 225–231
Olszewska MJ, Godlewski M (1972) An autoradiographic study of the synthesis of nucleic acids and protein during the cell cycle of synchronously dividing antheridial filaments inChara vulgaris L. Folia Histochem Cytochem 10: 245–256
Pickett-Heaps JD (1968) Ultrastructure and differentiation inChara sp. III. Formation of the antheridium. Aust J biol Sci 21: 255–274
Sussex IM, Clutter ME (1968) Differentiation in tissues, free cells and reaggregated plant cells. In vitro 3: 3–12
Thomson WW, Journett R de (1970) Studies on the ultrastructure of the guard cells ofOpuntia. Am J Bot 57: 309–220
Werker E, Vaughan JG (1974) Anatomical and ultrastructural changes in aleurone and myrosin cells ofSinapis alba during germination. Planta 116: 243–255
Author information
Authors and Affiliations
Additional information
This work is supported by the Polish Academy of Sciences within the project CPBP 04.01.5.05.
Rights and permissions
About this article
Cite this article
Kwiatkowska, M. Symplasmic isolation ofChara vulgaris antheridium and mechanisms regulating the process of spermatogenesis. Protoplasma 142, 137–146 (1988). https://doi.org/10.1007/BF01290870
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01290870