Summary
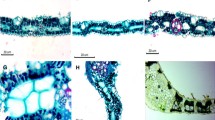
We investigated the histochemistry and ultrastructure of the cell walls of mestome sheaths and parenchymatous bundle sheaths of ten species of grasses. The species surveyed included representatives from all the major photosynthetic types: C3-Bromus tectorum, Phalaris arundinacea; C4/NAD-ME-Eragrostis cilianensis, Panicum capillare; C4/NAD-ME/PCK-Bouteloua curtipendula; C4/PCK-Chloris gayana, Sporobolus elongatus; C4/NADP-ME-Echinochloa crus-galli, Setaria glauca, Themeda triandra. All vein orders (designated here as major, minor and transverse) from mature leaves of each species were tested histochemically for lipids and phenols, and the majority of species were also examined with the electron microscope. A suberized lamella was detected ultrastructurally in at least some walls of major vein bundle sheath cells of all species examined. These lamellae were also present in some cells associated with the minor veins of the C3 species and in the minor and transverse veins of the C4/NADP-ME species. Histochemical tests for lipids and phenols consistently failed to differentiate this layer. Based on these tests, none of the vein orders in any species showed evidence of a Casparian band. In all suberized bundle sheaths, the compound middle lamella between cells with suberin lamellae is modified by the presence of phenols. These did not, however, confer resistance to acid digestion to the cell layer, in contrast to cell layers with Casparian bands. Therefore, although the mestome sheath has some features in common with the root endodermis (i.e. cells with a suberized lamella and thick, cellulosic walls which may be further modified), we could find no substantive anatomical or ultrastructural evidence for the presence of a Casparian band in any of the grass leaves investigated. The significance of these observations is discussed in the context of apoplastic permeability of these walls.
Similar content being viewed by others
References
Altus DP, Canny MJ (1985) Water pathways in wheat leaves. I. The division of fluxes between different vein types. Aust J Plant Physiol 12: 173–181
— —,Blackman DR (1985) Water pathways in wheat leaves. II Water-conducting capacities and vessel diameters of different vein types, and the behaviour of the integrated vein network. Aust J Plant Physiol 12: 183–199
Barnabas AD (1983) Composition and fine structural features of longitudinal veins in leaves ofThalassodendron ciliatum. S Afr J Bot 2: 317–325
Biggs AR (1984) Intracellular suberin: occurrence and detection in tree bark. IAWA Bulletin 5: 243–248
Blackman E (1971) The morphology and development of cross veins in the leaves of bread wheat (Triticum aestivum L.). Ann Bot 35: 653–665
Böcher TW, Oleson P (1978) Structural and ecophysiological pattern in the xero-halophytic C4 grassSporobolus rigens (Tr.). Desv Biolog Skrift 22: 3–48
Bonnett HT (1968) The root endodermis: fine structure and function. J Cell Biol 37: 199–205
Botha CEJ, Evert RF (1986) Free-space marker studies on the leaves ofSaccharum officinarum andBromus unioloides. S Afr J Bot 52: 335–342
— —,Cross RHM, Marshall DM (1982 a) The suberin lamella, a possible barrier to water movement from the veins to the mesophyll ofThemeda triandra Forsk. Protoplasma 112: 1–8
— — — — (1982 b) Comparative anatomy of matureThemeda triandra Forsk. leaf blades: a correlated light and electron microscope study. S Afr J Bot 48: 311–328
Bronner R (1975) Simultaneous demonstration of lipids and starch in plant tissues. Stain Technol 50: 1–4
Canny MJ (1986) Water pathways in wheat leaves. III. The passage of the mestome sheath and the function of the suberised lamellae. Physiol Plant 66: 637–647
Carolin RC, Jacobs SWL, Vesk M (1973) The structure of the cells of the mesophyll and parenchymatous bundle sheath of the Gramineae. Bot J Linn Soc 66: 259–275
Chonan N, Kawahara H, Matsuda T (1984) Ultrastructure of vascular bundles and fundamental parenchyma in relation to movement of photosynthate in leaf sheath of rice. Jap J Crop Sci 53: 435–444
— — — (1985) Ultrastructure of transverse veins in relation to phloem loading in the rice leaf. Jap J Crop Sci 54: 160–169
Clarkson DT, Robards AW, Sanderson J (1971) The tertiary endodermis in barley roots: fine structure in relation to radial transport of ions and water. Planta 96: 292–305
Dengler NG, Dengler RE, Hattersley PW (1985) Differing ontogenetic origins of PCR (“Kranz”) sheaths in leaf blades of C4 grasses (Poaceae). Am J Bot 72: 284–302
Eleftheriou EP, Tsekos I (1979) Development of mestome sheath cells in leaves ofAegilops comosa var.thessalica. Protoplasma 100: 139–153
Esau K (1977) Anatomy of seed plants, 2nd Ed. John Wiley and Sons Inc, New York, 550 pp
Espelie KE, Dean BB, Kolattukudy PE (1979) Composition of lipid derived polymers from different anatomical regions of several plant species. Plant Physiol 64: 1089–1093
—,Kolattukudy PE (1979) Composition of the aliphatic components of ‘suberin’ from the bundle sheaths ofZea mays leaves. Plant Sci Letters 15: 225–230
Eurenius L, Jarskar R (1970) A simple method to demonstrate lipids in Epon-embedded ultrathin sections. Stain Technol 45: 129–132
Evert RF, Botha CEJ, Mierza RJ (1985) Free-space marker studies in the leaf ofZea mays L. Protoplasma 126: 62–73
—,Eschrich W, Heyser W (1977) Distribution and structure of the plasmodesmata in mesophyll and bundle-sheath cells ofZea mays L. Planta 136: 77–89
— — — (1978) Leaf structure in relation to solute transport and phloem loading inZea mays L. Planta 138: 279–294
Griffith M, Huner NPA, Espelie KE, Kolattukudy PE (1985) Lipid polymers accumulate in the epidermal and mestome sheath cells during low temperature development of winter rye leaves. Protoplasma 125: 53–64
Guttenberg H von (1968) Der primÄre Bau der Angiospermenwurzel, Vol 8 In:Linsbauer K (ed) Handbuch der Pflanzenanatomie. Gebrüder Borntraeger, Berlin, pp 472
Haas DL, Carothers ZB (1975) Some ultrastructural observations on endodermal cell development inZea mays roots. Am J Bot 62: 336–348
Harris PJ, Hartley RD (1976) Detection of bound ferulic acid in the cell walls of the Gramineae by ultraviolet fluorescence microscopy. Nature 259: 508–510
— — (1980) Phenolic constituents of the cell walls of monocotyledons. Biochem System Ecol 8: 153–160
Hattersley PW, Browning AJ (1981) Occurrence of the suberized lamella in leaves of grasses of different photosynthetic types. I. In parenchymatous bundle sheaths and PCR (“Kranz”) sheaths. Protoplasma 109: 371–401
—,Perry S (1984) Occurrence of the suberized lamella in leaves of grasses of different photosynthetic type. II. In herbarium material. Aust J Bot 32: 465–473
—,Wong SC, Perry S, Roksandic Z (1986) Comparative ultrastructure and gas exchange characteristics of the C3-C4 intermediateNeurachne minor S.T. Blake (Poaceae). Plant, Cell Environ 9: 217–233
Holloway PJ (1983) Some variations in the composition of suberin from the cork layers of higher plants. Phytochemistry 22: 495–502
—,Brown CA, Browning AJ (1981) Ultrahistochemical detection of epoxides in plant cuticular membranes. J Exp Bot 32: 1051–1066
Huisinga B, Knijff AMW (1974) On the function of the Casparian strips in roots. Acta Bot Neerl 23: 171–175
Jensen WA (1962) Botanical histochemistry principles and practice. Freeman and Co, San Francisco-London, 408 pp
Johansen DA (1940) Plant microtechnique. McGraw-Hill Book Co, New York London, p 194
Kolattukudy PE (1978) Chemistry and biochemistry of the aliphatic components of suberin. In:Kahl G (ed) Biochemistry of wounded plant tissues. Walter de Gruyter, Berlin, pp 43–84
— (1980 a) Biopolyester membranes of plants: cutin and suberin. Science 208: 990–1000
— (1980 b) Cutin, suberin, and waxes. In:Stumpf PK (ed) The biochemistry of plants, Vol 4 Lipids: structure and function. Academic Press, New York, pp 571–567
— (1985) Biochemistry and function of cutin and suberin. Can J Bot 62: 2918–2933
Kuo J, O'Brien TP, Canny MJ (1974) Pit-field distribution, plasmodesmatal frequency, and assimilate flux in the mestome sheath cells of wheat leaves. Planta 121: 97–118
— —,Zee S-Y (1972) The transverse veins of the wheat leaf. Aust J Biol Sci 25: 721–737
Kutscha NP, Gray JR (1972) The suitability of certain stains for studying lignification in balsam fir,Abies balsamea (L.) Mill. Technical Bulletin 53: 1–50. Life Sciences and Agriculture Expt Stn, University of Maine, Orono
Laetsch WM (1969) Relationship between chloroplast structure and photosynthetic carbon fixation pathways. Sci Prog Oxf 57: 323–351
— (1971) Chloroplast structural relationships in leaves of C4 plants. In:Hatch MD, Osmond CB, Slayter RO (eds) Photosynthesis and photorespiration. Wiley Interscience, New York, pp 323–349
Lüttge U, Higinbotham N (1979) Transport in plants. Springer, Berlin Heidelberg New York, pp 84–85
Moon GJ, Peterson CA, Peterson RL (1984) Structural, chemical, and permeability changes following wounding in onion roots. Can J Bot 62: 2253–2259
O'Brien TP, Carr DJ (1970) A suberized layer in the cell walls of the bundle sheath of grasses. Aust J Biol Sci 23: 275–287
—,Feder N, McCully ME (1964) Polychromatic staining of plant cell walls by Toluidine Blue O. Protoplasma 59: 367–373
—,Kuo J (1975) Development of the suberized lamella in the mestome sheath of wheat leaves. Aust J Bot 23: 283–294
Pearse AGE (1968) Histochemistry theoretical and applied. Little, Brown and Co, Boston pp 691–692
Peirson DR, Dumbroff EM (1969) Demonstration of a complete Casparian strip inAvena andIpomoea by a fluorescent staining technique. Can J Bot 47: 1869–1871
Peterson CA, Emanuel ME, Humphreys GB (1981 a) Pathway of movement of apoplastic fluorescent dye tracers through the endodermis at the site of secondary root formation in corn (Zea mays) and broad bean (Vicia faba). Can J Bot 59: 618–625
— —,Weerdenberg CA (1981 b) The permeability of phi thickenings in apple (Pyrus malus) and geranium (Pelargonium hortorum) roots to an apoplastic fluorescent dye tracer. Can J Bot 59: 1107–1110
— —,Wilson C (1982) Identification of a Casparian band in the hypodermis of onion and corn roots. Can J Bot 60: 1529–1535
—,Griffith M, Huner NPA (1985) Permeability of the suberized mestome sheath in winter rye. Plant Physiol 77: 157–161
—,Perumalla CJ (1984) Development of the hypodermal Casparian band in corn and onion roots. J Expt Bot 35: 51–57
—,Peterson RL, Robards AW (1978) A correlated histochemical and ultrastructural study of the epidermis and hypodermis of onion roots. Protoplasma 96: 1–21
Prendergast HDV, Hattersley PW, Stone NE, Lazarides M (1986) C4 acid decarboxylation type inEragrostis (Poaceae): patterns of variation in chloroplast position, ultrastructure and geographical distribution. Plant, Cell Environ 9: 333–344
Priestly JH, North EE (1922) Physiological studies in plant anatomy. III. The structure of the endodermis in relation to its function. New Phytol 21: 113–139
Ramalingam K, Ravindranath MH (1970) Histochemical significance of green metachromasia to toluidine blue. Histochemistry 24: 322–327
Ride JP, Pearce RB (1979) Lignification and papilla formation at sites of attempted penetration of wheat leaves by non-pathogenic fungi. Physiol Plant Path 15: 79–92
Riley RG, Kolattukudy PE (1975) Evidence for covalently attached p-coumaric acid and ferulic acid in cutins and suberins. Plant Physiol 56: 650–654
Rufz de Lavison J de (1910) Du mode de pénétration de quelques sels dans la plante vivante Role de l'endoderme. Rev gén de Bot 22: 225–241
Russell SH, Evert RF (1985) Leaf vasculature inZea mays L. Planta 164: 448–458
Scalbert A, Monties B, Lallemand J-Y, Guittet E, Rolando C (1985) Ether linkage between phenolic acids and lignin fractions from wheat straw. Phytochemistry 24: 1359–1362
Schmidt HW, Schönherr J (1982) Fine structure of isolated and non-isolated potato tuber periderm. Planta 154: 76–80
Schwendener S (1890) Die Mestomscheiden der GramineenblÄtter. Sitzungsber Preuss Akad Wiss Phys-Math Kl 22: 405–426
Scott MG, Peterson RL (1979) The root endodermis inRanunculus acris. II. Histochemistry of the endodermis and the synthesis of phenolic compounds in roots. Can J Bot 57: 1063–1077
Spurr AR (1969) A low viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26: 31–43
Stroshine L, Cooke JR, Rand RH, Cutler JM, Chabot JF (1979) Mathematical analysis of pressure chamber efflux curves. Proc Amer Soc Agric Eng Paper No 79: 4585, pp 1–45
Strugger S (1940) Studien über den Transpirationsstrom im Blatt vonSecale cereale undTriticum vulgare. Z Bot 35: 97–113
Tippett JT, O'Brien TP (1976) The structure of eucalypt roots. Aust J Bot 24: 619–632
Van Fleet DS (1950) The cell forms, and their common substance reactions, in the parenchyma-vascular boundary. Bull Torr Bot Club 77: 340–353
Watson L, Dallwitz MJ (1980) Australian grass genera-anatomy, morphology, and keys. The Australian National University Research School of Biological Sciences, Canberra, pp 209
Weerdenberg CA, Peterson CA (1983) Structural changes in phi thickenings during primary and secondary growth in roots. 1. Apple (Pyrus malus) Rosaceae. Can J Bot 61: 2570–2576
Wilson JR, Hattersley PW (1983)In vitro digestion of bundle sheath cells in rumen fluid and its relationship to the suberized lamella and C4 photosynthetic type inPanicum species. Grass Forage Sci 38: 219–223
Wilson CA, Peterson CA (1983) Chemical composition of the epidermal, hypodermal, endodermal and intervening cortical cell walls of various plant roots. Ann Bot 51: 759–769
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Eastman, P.A.K., Dengler, N.G. & Peterson, C.A. Suberized bundle sheaths in grasses (Poaceae) of different photosynthetic types I. Anatomy, ultrastructure and histochemistry. Protoplasma 142, 92–111 (1988). https://doi.org/10.1007/BF01290867
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01290867