Summary
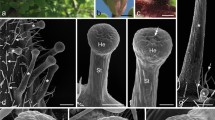
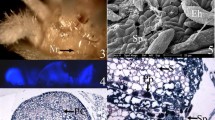
Nectary trichomes ofAbutilon striatum secrete copious amounts of sucrose, fructose and glucose. The nectar emerges from transient pores in the cuticle overlying the trichome tip cells. Calculations of the required transmembrane fluxes, either across the tip cell plasmalemma or across the cell membrane of the whole trichome, give very high rates compared with those obtained from other situations in plants and, therefore, cast doubt on the possibility that nectar secretion inAbutilon is an eccrine process. Quantitative evaluation of the possibility of granulocrine secretion, by successive fusion of vesicles with the cell membrane, suggests that this is an even less probable mechanism of secretion. Rapid freezing followed by freeze-substitution or freeze-fracture replication reveals that an extensive “secretory reticulum” (SR) is present within the hair cells. As similar micrographs are obtained from conventional, chemical fixation it is argued that the secretory reticulum is a relatively stable endomembrane system. Freeze-fracture and freeze-substitution micrographs show that this internal membrane system is closely associated with the plasmalemma. Taken together with other structural information, as well as physiological data, it is concluded that prenectar is actively loaded into the secretory reticulum of all trichome cells. Increase in hydrostatic pressure within this compartment leads to the opening of “sphincters” which connect the cisternal space of the SR to the outside of the plasmalemma. Thus a pulse of nectar is forcibly expelled into an apoplastic compartment sealed to the outside by the impermeable cuticle and on the inside by the plasmalemma. As this apoplastic compartment is also sealed at the stalk cell, the only route for pressure release is via the transient pores which overlay the tip cell. Distension renders these patent so that, again, pulsed secretion is observed. This hypothesis overcomes the necessity for envisaging excessively high transmembrane fluxes or rates of vesicle fusion. It would imply the need for a continuing supply of prenectar to the hair cells accompanied by active loading into the SR. This loading process may well be supported by the hydrolysis of sucrose to glucose and fructose and is probably the site where ions and other low molecular weight solutes are filtered from the nectar.
Similar content being viewed by others
References
De Maziere AMGL, Aertgeerts P, Dietrich W (1985) A modified cleansing procedure to obtain large freeze-fracture replicas. J Microsc 137: 185–188
Fahn A (1979) Secretory tissues in plants. Academic Press, London
Findlay N, Mercer FV (1971) Nectar production inAbutilon. 1 — Movement of nectar through the cuticle. Aust J Biol Sci 24: 647–656
— — (1971) Nectar production inAbutilon. 2 — Sub-microscopic structure of the nectary. Aust J Biol Sci 24: 657–664
—,Reed M, Mercer FV (1971) Nectar production inAbutilon. 3 — Sugar secretion. Aust J Biol Sci 24: 665–675
Gunning BES, Hughes JE (1976) Quantitative assessment of symplastic transport of pre-nectar into trichomes ofAbutilon nectaries. Aust J Plant Physiol 3: 619–637
Hughes JE (1977) Aspects of ultrastructure and function inAbutilon nectaries. ANU: M.Sc. Thesis.
Humbel B, Marti T, Muller M (1983) Improved structural preservation by combining freeze-substitution and low temperature embedding. Beitr elektronenmikroskop Direktabb Oberfl 16: 585
—,Müller M (1984) Freeze-substitution and low-temperatureembedding. Proc 8th Eur Reg Conf Electron Microscopy, Budapest 3: 1789
Kronestedt EC, Robards AW (1987) Sugar secretion from the nectary ofStrelitzia: an ultrastructural and physiological study. Protoplasma 137: 168–182
— —,Stark M, Olesen P (1986) Development of trichomes in theAbutilon nectary gland. Nordic J Bot 6: 627–639
Kushida H (1974) A new method for embedding with a low viscosity epoxy resin “Quetol 651”. J Electron Microscopy 23: 197
Loud AV (1967) Quantitative estimation of the loss of membrane images resulting from oblique sectioning of biological membranes. Proc 25th Ann Meeting Electron Microscope Society of America 144
Lüttge U (1964) Die Gewinnung und die chemische Analyse des Nektars. Deutsche Bienenwirtschaft 101–105
— (1977) Nectar composition and membrane transport of sugars and amino acids: a review on the present state of nectar research. Apidologie 8: 305–319
—,Schnepf E (1976) Organic substances. In:Lüttge U, Pitman MG (eds) Encyclopaedia of plant physiology, N.S. Transport in plants II. Part B, Tissue and Organs. Springer, Berlin Heidelberg New York, pp 244–277
McCully ME, Canny MJ (1985) The stabilization of labile configurations of plant cytoplasm by freeze-substitution. J Microscopy 139: 27–33
Mersey B, McCully ME (1978) Monitoring the course of fixation of plant cells. J Microscopy 114: 49–84
Reed ML, Findlay N, Mercer FV (1971) Nectar production inAbutilon. 4 — Water and solute relations. Aust J Biol Sci 24: 677–688
Robards AW (1984) Fact or artefact—a cool look at biological electron microscopy. Proc Roy microsc Soc (Presidential address to the Microscopical Society) 19: 195–208
— (1985) The use of low temperature methods for structural and analytical studies of plant transport processes. In:Robards AW (ed) Botanical microscopy 1985. Oxford University Press, Oxford, pp 39–64
—,Crosby P (1983) Optimisation of plunge-freezing: linear relationship between cooling rate and entry velocity into liquid propane. Cryo-Letters 4: 23–32
—,Clarkson DT (1984) Effects of chilling temperature on root cell membranes as viewed by freeze-fracture electron microscopy. Protoplasma 122: 75–85
-Sleytr UB (1985) Low temperature methods in biological electron microscopy. In:Glauert AM (ed) Practical methods in electron microscopy. North-Holland/Elsevier
—,Oates K (1986) X-ray microanalysis of ion distribution inAbutilon nectary hairs. J exp Bot 37: 940–946
Sleytr UB, Umrath W (1974) A simple fracturing device for obtaining complementary replicas of freeze-fractured and freeze-etched suspensions and tissue fragments. J Microscopy 101: 177
Terry BR, Robards AW (1987) Hydrodynamic radius alone governs the mobility of molecules through plasmodesmata. Planta 171: 145–157
Unzelman JM, Healey PL (1974) Development, structure and occurrence of secretory trichomes ofPharbitis. Protoplasma 80: 285–303
Williams MA (1977) Quantitative methods in biology. In:Glauert AM (ed) Practical methods in electron microscopy. North-Holland, Amsterdam, pp 234
Willison JHM (1976) Plasmodesmata: a freeze-fracture view. Can J Bot 54: 2842–2847
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Robards, A.W., Stark, M. Nectar secretion inAbutilon: a new model. Protoplasma 142, 79–91 (1988). https://doi.org/10.1007/BF01290866
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01290866