Summary
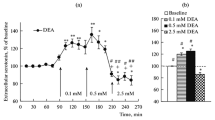
The selective serotonin uptake inhibitor fluoxetine (10mg/kg i.p.) increased tissue levels of the norepinephrine metabolite 3-methoxy-4-hydroxyphenylethylene glycol sulfate (MHPG-SO4) in rat hypothalamus, indicating an increased release of norepinephrine. Microdialysis studies in conscious rats showed that fluoxetine (10 mg/kg i.p.) increased extracellular concentrations of norepinephrine as well as serotonin in the hypothalamus. In contrast, desipramine (10mg/kg i.p.) increased extracellular concentration of norepinephrine but not serotonin in the hypothalamus. Consistent with its mechanism of being a selective serotonin uptake inhibitor, local perfusion of fluoxetine (10μM) caused a 7-fold increase in hypothalamic extracellular serotonin and a small non-significant increase in extracellular norepinephrine. The subsequent systemic injection of fluoxetine (10mg/kg s.c.) after local perfusion caused a 3-fold increase in extracellular norepinephrine, indicating that fluoxetine's action leading to an increase in extracellular norepinephrine was not occurring in the terminal areas of the hypothalamus but elsewhere in the brain, possibly cell bodies in the locus coeruleus.
Similar content being viewed by others
References
Bareggi SR, Markey K, Genovese E (1978) Effects of single and multiple doses of desipramine on endogenous levels of 3-methyoxy-4-hydroxyphenylglycol-sulfate (MOPEG-SO4) in rat brain. Eur J Pharmacol 50: 301–306
Blandina P, Goldfarb J, Walcott J, Green JP (1991) Serotonergic modulation of the release of endogenous norepinephrine from rat hypothalamic slices. J Pharmacol Exp Ther 256: 341–347
Calderini G, Morselli PL, Garattini S (1975) Effect of amphetamine and fenfluramine on brain noradrenaline and MOPEG-SO4. Eur J Pharmacol 34: 345–350
Cedarbaum JM, Aghajanian GK (1978) Afferent projections to the rat locus coeruleus as determined by a retrograde tracing technique. J Comp Neurol 178: 1–16
Chen NH, Reith MEA (1995) Monoamine interactions measured by microdialysis in the ventral tegmental area of rats treated systemically with (±)-8-hydroxy-2-(di-n-proplyamino)tetralin. J Neurochem 64: 1585–1597
Clement HW, Gemsa D, Wesmann W (1992) Serotonin-norepinephrine interactions: a voltammetric study on the effect of serotonin receptor stimulation followed in the N raphe dorsalis and the locus coeruleus of the rat. J Neural Transm [GenSect] 88: 11–23
Crawley JN, Mass JW, Roth RH (1980) Biochemical evidence for simultaneous activation of multiple locus coeruleus efferents. Life Sci 26: 1373–1378
Done CJ, Sharp T (1994) Biochemical evidence for the regulation of central noradrenergic activity by 5-HT1A and 5-HT2 receptors: microdialysis studies in the awake and anaesthetized rat. Neuropharmacology 33: 411–421
Feuerstein TJ, Hertting G (1986) Serotonin (5-HT) enhances hippocampal noradrenaline (NA) release: evidence for facilitatory 5-HT receptors within the CNS. Naunyn Schmiedebergs Arch Pharmacol 333: 191–197
Fuller RW (1994) Uptake inhibitors increase extracellular serotonin concentration measured by brain microdialysis. Life Sci 55: 163–167
Fuller RW, Perry KW (1989) Effects of buspirone and its metabolite, 1-(2-pyrimidinyl)piperazine, on brain monoamines and their metabolites in rats. J Pharmacol Exp Ther 248: 50–56
Gariepy KC, Bailey B, Yu J, Maher T, Ackworth IN (1994) Simultaneous determination of norepinephrine, dopamine, and serotonin in hippocampal microdialysis samples using normal bore high performance liquid chromatography: effects of dopamine receptor agonist stimulation and euthanasia. J Liquid Chromatogr 17: 1541–1556
Goldfarb J, Walcott J, Blandina P (1993) Serotonergic modulation of L-glutamic acidevoked release of endogenous norepinephrine from rat hypothalamus. J Pharmacol Exp Ther 267: 45–50
Hemrick-Luecke SK, Snoddy HD, Fuller RW (1994) Evaluation of nefazodone as a serotonin uptake inhibitor and serotonin antagonist in vivo. Life Sci 55: 479–483
Herve D, Pickel VM, Joh TH, Beaudet A (1987) Serotonin axon terminals in the ventral tegmental area of the rat: fine structure and synaptic input to dopaminergic neurons. Brain Res 435: 71–83
Hjorth S, Carlsson A (1986) Is pindolol a mixed agonist-antagonist at central serotonin(5-HT) receptors? Eur J Pharmacol 129: 131
Jordan S, Kramer GL, Zukas PK, Moeller M, Petty F (1994) In vivo biogenic amine efflux in medial prefrontal cortex with imipramine, fluoxetine, and fluvoxamine. Synapse 18: 294–297
Leger L, Descarries L (1978) Serotonin nerve terminals in the locus coeruleus of adult rat: a radioautographic study. Brain Res 145: 1–13
Mayle DA, Robertson DW, Wong DT (1991) Elevation of catecholaminergic metabolite levels in the rat hypothalamus by an agonist of serotonin 1C/2 receptors, MK212 (6-chloro-2-(l-piperazinyl) pyrazine). Neurosci Abstr 17: 407
McRae-Degueurce A, Dennis T, Leger L, Scatton B (1985) Regulation of noradrenergic neuronal activity in the rat locus coeruleus by serotonergic afferents. Physiol Psychol 13: 188–196
Mongeau R, De Montigny C, Blier P (1994) Activation of 5-HT3 receptors enhances the electrically evoked release of [3H]noradrenaline in rat brain limbic structures. Eur J Pharmacol 256: 269–279
Paez X, Leibowitz S (1993) Changes in extracellular PVN monoamines and macronutrient intake after idazoxan or fluoxetine injection. Pharm Biochem Behav 46: 933–941
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates. Academic Press, San Diego CA
Perry KW, Fuller RW (1991) Serotonergic drugs increase MHPG-SO4 in rat hypothalamus. Neurosci Abstr 17: 1178
Perry KW, Fuller RW (1992) Effect of fluoxetine on serotonin and dopamine concentration in microdialysis fluid from rat striatum. Life Sci 50: 1683–1690
Pickel VM, Joh TH, Chan J, Beaudet A (1984) Serotonergic terminals: ultrastuctural and synaptic interaction with catecholamine-containing neurons in the medial nuclei of the solitary tracts. J Comp Neurol 225: 291–301
Pozzi L, Invernizzi R, Cervi L, Vallebuona F, Samanin R (1994) Evidence that extracellular concentrations of dopamine are regulated by noradrenergic neurons in the frontal cortex of rats. J Neurochem 63: 195–200
Rutter JJ, Auerbach SB (1993) Acute uptake inhibition increases extracellular serotonin in the rat forebrain. J Pharmacol Exp Ther 265: 1319–1324
Sabol SE, Richards JB, Sciden LS (1992) Fenfluramine-induced increases in extracellular hippocampal serotonin are progressively attenuated in vivo during a four-day fenfluramine regimen in rats. Brain Res 571: 64–72
Scatton B, Claustre Y, Graham D, Dennis T, Serrano A, Arbilla S, Pimoule C, Schoemaker H, Bigg D, Langer SZ (1988) SL 81 0385: a novel selective and potent serotonin uptake inhibitor. Drug Dev Res 12: 29–40
Smythe GA, Gleeson RM, Stead BM (1988) Mechanisms of 5-hydroxy-L-tryptophaninduced adrenocorticotropin release: a major role for central noradrenergic drive. Neuroendocrinology 47: 389–397
Suzuki M, Matsuda T, Asano S, Somboonthum P, Takuma K, Baba A (1995) Increase of noradrenaline release in the hypothalamus of freely moving rat by postsynaptic 5-hydroxytryptamine(la) receptor activation. Br J Pharmacol 115: 703–711
Tanda G, Carboni E, Frau R, Di Chiara G (1994) Increase in extracellular dopamine in the prefrontal cortex: a trait of drugs with antidepressant potential? Psychopharmacology 115: 285–288
Tian Y, Eaton MJ, Goudreau JL, Lookingland KJ, Moore KE (1993) Neurochemical evidence that 5-hydroxytryptaminergic neurons tonically inhibit noradrenergic neurons terminating in the hypothalamus. Brain Res 607: 215–221
Weissmann-Nanopoulos D, Mach E, Magre J, Demassey Y, Pujo J (1985) Evidence for the localization of 5HT1A binding sites on serotonin containing neurons in the raphe dorsalis and raphe centralis nuclei of the rat brain. Neurochem Int 7: 1061–1072
Wong DT, Bymaster FP, Reid LR, Threlkeld PG (1983) Fluoxetine and two other serotonin uptake inhibitors without affinity for neuronal receptors. Biochem Pharmacol 32: 1287–1293
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Perry, K.W., Fuller, R.W. Fluoxetine increases norepinephrine release in rat hypothalamus as measured by tissue levels of MHPG-SO4 and microdialysis in conscious rats. J. Neural Transmission 104, 953–966 (1997). https://doi.org/10.1007/BF01285563
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01285563