Summary
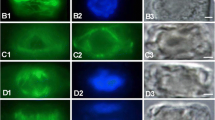
Studies of monoplastidic mitosis in hornworts (Bryophyta) using transmission electron microscopy and indirect immunofluorescence staining of microtubules have revealed that two mutually perpendicular microtubule systems predict division polarity in preprophase. Events of cytoplasmic reorganization in preparation for division occur in the following order: migration of the single plastid to a position perpendicular to the division site, constriction of the plastid where its midpoint intersects the division site, development of an axial system of microtubules parallel to the elongating plastid isthmus, and appearance of an atypical preprophase band of microtubules (PPB). The PPB is asymmetrical with a tight band of microtubules on the side over the plastid isthmus and a broad band of widely spaced microtubules over the nucleus. The axial system contributes directly to development of the spindle. In prometaphase, the axial system separates at the equator and additional microtubule bundles project from polar regions, creating two opposing halfspindles. The PPB is still present during asymmetrical organization of the spindle and microtubules extending from the broad portion of the PPB to poles appear to be incorporated into the developing spindle. Dynamic changes in the microtubular cytoskeleton demonstrate (1) intimate relationship of plastid and nuclear division, (2) contribution of preprophase/prophase microtubule systems to spindle development in monoplastidic cells, and (3) dynamic reorientation of microtubules from one system to another.
Similar content being viewed by others

References
Apostolakos P, Galatis B (1985) Studies on the development of the air pores and air chambers ofMarchantia paleacea III. Microtubule organization in preprophase-prophase initial aperture cells-formation of incomplete preprophase microtubule bands. Protoplasma 128: 120–135
Brown RC, Lemmon BE (1984) Plastid apportionment and preprophase microtubule bands in monoplastidic root meristem cells ofIsoetes andSelaginella. Protoplasma 123: 95–103
— (1985 a) Preprophasic establishment of division polarity in monoplastidic mitosis of hornworts. Protoplasma 124: 175–183
— (1985 b) Development of stomata inSelaginella. Division polarity and plastid movements. Am J Bot 72: 1914–1925
— (1985 c) A cytoskeletal system predicts division plane in meiosis ofSelaginella. Protoplasma 127: 101–109
— (1987 a) Division polarity, development and configuration of microtubule arrays in bryophyte meiosis I. Meiotic prophase to metaphase I. Protoplasma 137: 84–99
— (1987 b) Division polarity, development and configuration of microtubule arrays in bryophyte meiosis II. Anaphase I to the tetrad. Protoplasma 138: 1–10
Buchen B, Sievers A (1981) Sporogenesis and pollen grain formation. In:Kiermayer O (ed) Cytomorphogenesis in plants. Springer, Wien New York, pp 349–376
Busby CH (1986) Development of the meiotic cytoskeleton in bryophytes. MS Thesis, Australian National University, Canberra
Calarco-Gillam PD, Siebert MC, Hubble R, Mitchison T, Kirschner M (1983) Centrosome development in early mouse embryos as defined by an auto-antibody against pericentriolar material. Cell 35: 621–629
Clayton L, Black CM, Lloyd CW (1985) Microtubule nucleating sites in higher plant cells identified by an auto-antibody against pericentriolar material. J Cell Biol 101: 319–324
Crandall-Stotler B (1980) Morphogenetic designs and a theory of bryophyte origins and divergence. Bioscience 30: 580–585
Doonan JH, Cove DJ, Lloyd CW (1985) Immunofluorescence microscopy of microtubules in intact cell lineages in the moss,Phycomitrella patens I. Normal and CIPC-treated tip cells. J Cell Sci 75: 131–147
—,Cove DJ, Corke FMK, Lloyd CW (1987) Pre-prophase band of microtubules, absent from tip-growing moss filaments, arises in leafy shoots during transition to intercalary growth. Cell Motil Cytoskel 7: 138–153
Dunlop DW (1949) Notes on the cytology of some lycopsids. Bull Torrey Bot Club 76: 266–277
Euteneuer U, McIntosh JR (1980) Polarity of midbody and phragmoplast microtubules. J Cell Biol 87: 509–515
Fowke LC, Pickett-Heaps JD (1978) Electron microscope study of vegetative cell division in two species ofMarchantia. Can J Bot 56: 467–475
Galatis B, Apostolakos P (1977) On the fine structure of differentiating mucilage papillae ofMarchantia. Can J Bot 55: 772–795
Gunning BES (1982) The cytokinetic apparatus: Its development and spatial regulation. In:Lloyd CW (ed) The cytoskeleton in plant growth and development. Academic Press, London New York, pp 229–292
Lander CA (1935) The relation of the plastid to nuclear division inAnthoceros laevis. Am J Bot 22: 42–51
Marchant HJ, Pickett-Heaps JD (1973) Mitosis and cytokinesis inColeochaete scutata. J Phycol 9: 461–471
McIntosh JR (1981) Microtubule polarity and interaction in mitotic spindle function. In:Schweiger HG (ed) International cell biology 1980–1981. Springer, Berlin Heidelberg New York, pp 359–368
—,Euteneuer U (1984) Tubulin hooks as probes for microtubule polarity: an analysis of the method and an evaluation of data on microtubule polarity in the mitotic spindle. J Cell Biol 98: 525–533
Mitchison T, Kirschner M (1984) Microtubule assembly nucleated by isolated centrosomes. Nature (London) 312: 232–236
Mole-Bajer J, Baser AS (1968) Studies of selected endosperm cells with the light and electron microscope. The technique. Cellule 67: 257–265 + 5 pl
Pickett-Heaps JD (1967) Ultrastructure and differentiation inChara sp. I. Vegetative cells. Aust J Biol Sci 20: 539–551
— (1974) Plant microtubules. In:Robards AW (ed) Dynamic aspects of plant ultrastructure. McGraw-Hill, London New York, pp 219–255
—,Tippit DH, Cohn SA, Spurck TP (1986) Microtubule dynamics in the spindle. Theoretical aspects of assembly/disassembly reactionsin vivo. J Theor Biol 118: 153–169
Sack FD, Paollilo DJ (1985) Incomplete cytokinesis inFunaria stomata. Am J Bot 72: 1325–1333
Schliwa M, van Blerkom J (1981) Structural interaction of cytoskeletal components. J Cell Biol 90: 222–235
Schmiedel G, Reiss H-D, Schnepf E (1981) Associations between membranes and microtubules during mitosis and cytokinesis in caulonema tip cells of the mossFunaria hygrometrica. Protoplasma 108: 173–190
Schnepf E(1973) Mikrotubulus-Anordnung und -Umordnung, Wandbildung und Zellmorphogenese in jungenSphagnum-Blättchen. Protoplasma 78: 145–173
Schroeder M, Wehland J, Weber K (1985) Immunofluorescence microscopy of microtubules in plant cells; stabilization by dimethylsulfoxide. Eur J Cell Biol 38: 211–218
Steer MW (1984) Mitosis in Bryophytes. AdvBryol 2: 1–23 + 27 figs
Telzer BR, Haimo LT (1981) Decoration of spindle microtubules with dynein: evidence for uniform polarity. J Cell Biol 89: 373–378
Wick SM (1985) Immunofluorescence microscopy of tubulin and microtubule arrays in plant cells. III. Transition between mitotic/ cytokinetic and interphase microtubule arrays. Cell Biol Int Rep 9: 357–371
—,Duniec J (1983) Immunofluorescence microscopy of tubulin and microtubule arrays in plant cells. I. Preprophase band development and concomitant appearance of nuclear envelope-as-sociated tubulin. J Cell Biol 97: 235–243
— — (1984) Immunofluorescence microscopy of tubulin and microtubule arrays in plant cells. II. Transition between the preprophase band and the mitotic spindle. Protoplasma 122: 45–55
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brown, R.C., Lemmon, B.E. Preprophasic microtubule systems and development of the mitotic spindle in hornworts (Bryophyta). Protoplasma 143, 11–21 (1988). https://doi.org/10.1007/BF01282954
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01282954