Conclusions
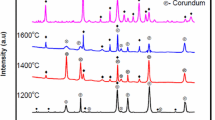
It is shown that, depending on the composition of the original body, with the same cooling conditions for the melt, crystallization of mullite develops in three different types distinguished by structure, chemical, and phase composition.
In the OKB-514 arc furnace, using a voltage of 194 V and a current of 1800 A it is possible to synthesize mullite, whose composition is slightly different from that of the orginal mixture. The use of lower voltages causes slight changes in the compositon of the fused materials on account of the reduction of part of the SiO2 and the resulting materials of mullite-corundum composition.
It is found that the loss of SiO2 during fusing occurs mainly in the initial period of melting of silica-containing constituent of the mixture. Coarsening the grain-size composition of this component leads to an increased loss of silica and corundum enrichment of the melt.
The ingots of molten material contain crystallization structures that vary in relation to the alumina modulus. When Al2O3/SiO2<3.5 crystallization of mullite has a directional character, moving from the cooling surface to the center of the ingot. When Al2O3/SiO2 is from 3.5 to 4.5 the ingot develops a zonal structure; in the lower part corundum crystallizes with mullite; in the upper zone we note directional crystallization of mullite. When A12O3/ SiO2>4.5 the ingot forms fine-grained structures from grains of corundum of prismatic and isometric form and also mullite.
Similar content being viewed by others
Literature cited
P. Cichy, Interceram.,24, No. 4, 293–296 (1974).
E. Frishbutter, H. Kleist, and W. Schroder, Silikattechnik, No. 11, 369–372 (1969).
V. P. Elyutin, Yu. A. Pavlov, et al., Production of Ferroalloys [in Russian], Moscow (1957), p. 436.
Ya. S. Shchedrovitskii, Complex Silicon Ferroalloys [in Russian], Metallurgiya, Moscow (1966), p. 176.
N. A. Toropov and F. Ya. Galakhov, Dokl. Akad. Nauk SSSR,78, No. 2, 299–302 (1956).
R. R. Barta and U. R. Barta, Zh. Prikl. Khim.,29, No. 3, 341–353 (1956).
N. M. Galdina, Ogneupory, No. 12, 540–548 (1951).
A. N. Sokolov, I. V. Shurygina, S. P. Shmitt-Fogelevich, et al., Ogneupory, No. 9, 35–39 (1979).
L. I. Karyakin, Mineralogical Handbook of I. Franko L'vov State University, L'vov, Vishcha Shkola, No. 1, 27, 66–78 (1973).
H. Lorens, Silikattechnik,25, No. 8, 274–278 (1974).
N. V. Pitak, T. A. Ansimova, Ogneupory, No. 1, 35–38 (1974).
Reaction of Metal Oxides and Carbon [in Russian], V. P. Elyutin, Yu. A. Pavlov, V. P. Polyakov, et al., Metallurgiya, Moscow (1976), p. 360.
L. A. Korobka, V. S. Shapovalov, and V. A. Ustichenko, Ogneupory, No. 12, 44–47 (1978).
Author information
Authors and Affiliations
Additional information
Translated from Ogneupory, No. 9, pp. 21–28, September, 1990.
Rights and permissions
About this article
Cite this article
Ustichenko, V.A., Pitak, N.V. & Shapovalov, V.S. Influence of technological factors on obtaining ingots of mullite and mullite-corundum compositions. Refractories 31, 502–510 (1990). https://doi.org/10.1007/BF01282780
Issue Date:
DOI: https://doi.org/10.1007/BF01282780