Summary
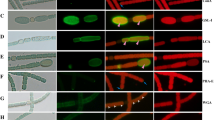
Geosiphon pyriforme represents a photoautotrophic endosymbiosis of aGlomus-like fungus with the cyanobacteriumNostoc punctiforme. The fungus forms unicellular bladders of up to 2 mm in length and 0.5 mm in diameter growing on the soil surface and harboring the endosymbioticNostoc filaments. The cyanobacteria are located in a compartment (the symbiosome) bordered by a host membrane. The space between this symbiosome membrane (SM) and theNostoc cell wall is filled with an about 30–40 nm thick layer of amorphous material, which is present also in the regions of the symbiosome where noNostoc filaments are located. At these sites the amorphous material consists of a 20–30 nm thick layer separating the SM. The region between the SM and the cyanobacterium is defined as symbiosome space (SS). Fungal bladders, hyphae and free livingNostoc were analyzed by affinity techniques as well as the material occurring in the SS. FITC-coupled lectins with sugar specificity to α-D-mannosyl/α-D-glucosyl (Con A), N-acetyl-β-D-glucosamine oligomers (WGA), α-L-fucosyl (UEA-I), β-D-galactosyl (RCA-120), α-D-galactosyl (BS-I-B4), N-acetyl-α-D-galactosamine (HPA), and sialic acid (EBL) residues were tested. WGA binding and calcofluor white staining demonstrated that the bladder wall as well as the SS contain fibrillar chitin. Of the other lectins only Con A clearly labeled the symbiosome. On the contrary, the lectin binding properties of the slime produced by free livingNostoc-colonies indicate the presence of mannose, fucose, GalNAc, sialic acid, and galactose, while chitin or GlucNAc-oligomers could not be detected. The symbiosome was also investigated electron microscopically. WGA-gold binding confirmed the presence of chitin, while a slight PATAg reaction indicated some polysaccharidic molecules within the SS. Our results show that the amorphous material within the SS contains molecules typical of the fungal cell wall and suggest that the SM is related to the fungal plasma membrane. The applied lectins all bind to the hyphal surface, indicating a high molecular complexity. Mannosyl, β-galactosyl, and sialic acid residues are strongly exposed at the outer cell wall layer, whereas GlucNAc, GalNAc, and α-galactosyl residues seem to be present in smaller amounts. The symbiotic interface established between the fungus andNostoc inGeosiphon shows many similarities to that occurring between fungi and root cells in arbuscular mycorrhizas.
Similar content being viewed by others
Abbreviations
- AM:
-
arbuscular mycorrhiza
- BS-I-B4 :
-
Bandeiraea simplicifolia lectin I isolectin B4
- CLSM:
-
confocal laser scanning microscopy
- Con A:
-
Concanavalin A
- EBL:
-
elderberry bark lectin I
- FITC:
-
fluorescein isothiocyanate
- HPA:
-
Helix pomatia agglutinin
- PATAg:
-
periodic acid-thiocarbohydrazide-Ag proteinate
- SM:
-
symbiosome membrane
- SS:
-
symbiosome space
- RCA-120:
-
Ricinus communis agglutinin 120
- UEA-I:
-
Ulex europaeus agglutinin I
- WGA:
-
wheat germ agglutinin
References
Bartnicki-Garcia S (1987) The cell wall: a crucial structure in fungal evolution. In: Rayner ADM, Brasier CM, Moore D (eds) Evolutionary biology of the fungi. Cambridge University Press, Cambridge, pp 389–403
Bishop CT, Adams GA, Hughes EO (1954) A polysaccharide from the blue-green alga,Anabaena cylindrica. Can J Chem 32: 999–1004
Bonfante P (1994) Ultrastructural analysis reveals the complex interactions between root cells and arbuscular mycorrhizal fungi. In: Gianinazzi S, Schüepp H (eds) Impact of arbuscular mycorrhizas on sustainable agriculture and natural ecosystems. Birkhäuser, Basel, pp 73–87
—, Bianciotto V (1995) Saprotrophic versus symbiotic phase in endomycorrhizal fungi: morphology and cytology. In: Hoch B, Varma A (eds) Mycorrhiza: structure, function, molecular biology and biotechnology. Springer, Berlin Heidelberg New York Tokyo, pp 229–247
Bonfante P, Grippiolo R (1984) Cytochemical and biochemical observations on the cell wall of the spores ofGlomus epigaeum. Protoplasma 123: 140–151
—, Perotto S (1995) Strategies of colonization by arbuscular mycorrhizal fungi when infecting host plants. New Phytol 130: 3–21
—, Spanu P (1992) Pathogenic and endomycorrhizal associations. Methods Microbiol 24: 141–168
—, Perotto S, Testa B, Faccio A (1987) Ultrastructural localization of cell surface sugar residues in ericoid mycorrhizal fungi by gold-labeled lectins. Protoplasma 139: 25–35
—, Faccio A, Perotto S, Schubert A (1990) Correlation between chitin distribution and cell wall morphology in the mycorrhizal fungusGlomus versiforme. Mycol Res 94: 157–165
Deacon JW (1984) Introduction to modern mycology: structure and fine structure. In: Wilkinson JF (ed) Basic microbiology, vol 7. Blackwell, Oxford, pp 32–35
Dunn JH, Wolk CP (1970) Composition of the cellular envelopes ofAnabaena cylindrica. J Bacteriol 103: 153–158
Grosskopf DG, Kroiher M (1988) Gradients in plastid and mitochondrial DNA synthesis inFunaria protonemata: visualisation by bromodeoxyuridine immunohistochemistry. Protoplasma 147: 1–4
Guillot J, Breton A, Damez M, Dusser M, Gaillard-Martinie B, Millet L (1990) Use of lectins for a comparative study of cell wall composition of different anaerobic rumen fungal strains. FEMS Microbiol Lett 67: 151–156
Herth W, Schnepf E (1980) The fluorochrome, calcofluor white, binds oriented to structural polysaccharide fibrils. Protoplasma 105: 129–133
Hill DR, Peat A, Potts M (1994) Biochemistry and structure of the glucan secreted by desiccation-tolerantNostoc commune (Cyanobacteria). Protoplasma 182: 126–148
Höchst H, Martin HH, Kandier O (1965) Zur Kenntnis der chemischen Zusammensetzung der Zellwand der Blaualgen. Z Pflanzenphysiol 53: 39–57
Hough L, Jones JKN, Wadman WH (1952) An investigation of the polysaccharide components of certain fresh-water algae. J Chem Soc 1952: 3393–3399
Jabaji-Hare SH, Thérien J, Charest PM (1990) High resolution cytochemical study of the vesicular-arbuscular mycorrhizal association,Glomus darum xAllium porrum. New Phytol 114: 481–496
Johnson GD, Araujo GM, de Nogueira C (1981) A simple method of reducing the fading of immunofluorescence during microscopy. J Immunol Methods 43: 349–350
Kluge M, Mollenhauer D, Mollenhauer R (1991) Photosynthetic carbon assimilation inGeosiphon pyriforme (Kützing) F v Wettstein, an endosymbiotic association of fungus and cyanobacterium. Planta 185: 311–315
— — —, Kape R (1992)Geosiphon pyriforme, an endosymbiotic consortium of a fungus and a cyanobacterium (Nostoc), fixes nitrogen. Bot Acta 105: 343–344
— — — (1994)Geosiphon pyriforme (Kützing) von Wettstein, a promising system for studying endocyanoses. Prog Bot 55: 130–141
Knapp E (1933) ÜberGeosiphon pyriforme Fr. Wettst, eine intrazelluläre Pilz-Algen-Symbiose. Ber Dtsch Bot Ges 51: 210–217
Kokyrsta PN, Chekoi VN (1972) Biochemical composition of the blue-green algaNostoc linckia f.muscorum (AG) Elenk. and its lifelong production. Ser Biol Khim 2: 46–48 (in Russian)
Martin C, Codd GA, Siegelman HW, Weckesser J (1989) Lipopolysaccharides and polysaccharides of the cell envelope of toxicMicrocystis aeruginosa strains. Arch Microbiol 152: 90–94
Mehta VB, Vaidya BS (1978) Cellular and extracellular polysaccharides of the blue-green algaNostoc. J Exp Bot 29: 1423–1430
Mollenhauer D (1988) Weitere Untersuchungen anGeosiphon pyriforme-einer Lebensgemeinschaft von Pilz und Blaualge. Natur Museum 118: 289–309
— (1992)Geosiphon pyriforme. In: Reisser W (ed) Algae and symbiosis: plants, animals, fungi, viruses, interactions explored. Biopress, Bristol, pp 339–351
—, Mollenhauer R (1988)Geosiphon cultures ahead. Endocytobios Cell Res 5: 69–73
—, Kluge M (1994)Geosiphon pyriforme. Endocytobios Cell Res 10: 29–34
Moore BG, Tischer RG (1964) Extracellular polysaccharides of algae: effects on life-support systems. Science 145: 586–587
— — (1965) Biosynthesis of extracellular polysaccharides by the blue-green algaAnabaena flos-aquae. Can J Microbiol 11: 877–885
Morton JB, Benny GL (1990) Revised classification of arbuscular mycorrhizal fungi (Zygomycetes): a new order, Glomales, two new suborders, Glomineae and Gigasporineae, and two new families, Acaulosporaceae and Gigasporaceae, with an emendation of Glomaceae. Mycotaxon 37: 471–491
Pritzer M, Weckesser J, Jürgens UJ (1989) Sheath and outer membrane components from the cyanobacteriumFischerella sp. PCC 7414. Arch Microbiol 153: 7–11
Roland JC (1978) General preparation and staining of thin sections. In: Hall JL (ed) Electron microscopy and cytochemistry of plant cells. North-Holland, Amsterdam, pp 1–62
Roth LE, Jean K, Stacey G (1988) Homology in endosymbiotic systems: the term “symbiosome”. In: Palacios R, Verma DPS (eds) Molecular genetics of plant-microbe interactions. The American Phytopathological Society Press, St Paul, MN, p 220
Sangar VK, Dugan PR (1972) Polysaccharide produced byAnacystis nidulans: its ecological implication. Appl Microbiol 24: 732–734
Schneider S, Jürgens U (1991) Cell wall and sheath constituents of the cyanobacteriumGloeobacter violaceus. Arch Microbiol 156: 312–318
Schnepf E (1964) Zur Feinstruktur vonGeosiphon pyriforme. Arch Microbiol 49: 112–131
Schrader M, Drews G, Golecki JR, Weckesser J (1982) Isolation and characterization of the sheath from the cyanobacteriumChlorogloeopsis PCC 6912. J Gen Microbiol 128: 267–272
Schüßler A, Mollenhauer D, Schnepf E, Kluge M (1994)Geosiphon pyriforme, an endosymbiotic association of fungus and cyanobacteria: the spore structure resembles that of arbuscular mycorrhizal (AM) fungi. Bot Acta 107: 36–45
—, Schnepf E, Mollenhauer D, Kluge M (1995) The fungal bladders of the endocyanosisGeosiphon pyriforme, a Glomus-related fungus: cell wall permeability indicates a limiting pore radius of only 0.5 nm. Protoplasma 185: 131–139
Sengbusch P v, Müller U (1983) Distribution of glycoconjugates at algal surfaces as monitored by FITC-conjugated lectins. Studies on selected species from Cyanophyta, Pyrrophyta, Raphidophyta, Euglenophyta, Chromophyta, and Chlorophyta. Protoplasma 114: 103–113
Simon L, Bousquet J, Lévesque RC, Lalonde M (1993) Origin and diversification of endomycorrhizal fungi and coincidence with vascular land plants. Nature 363: 67–69
Smith SE, Smith FA (1990) Structure and function of the interfaces in biotrophic symbioses as they relate to nutrient transport. New Phytol 114: 1–38
Tease BE, Walker RW (1987) Comparative composition of the sheath of the cyanobacteriumGloeothece ATCC 27152 cultured with and without combined nitrogen. J Gen Microbiol 133: 3331–3339
Tischer RG, Moore BG (1964) An extracellular polysaccharide produced byPalmella mucosa Kütz. Arch Mikrobiol 49: 158–166
van der Merwe PA, Barclay AN (1994) Transient intercellular adhesion: the importance of weak protein-protein interactions. Trend Biochem Sci 19: 354–358
Vincenzini M, De Phillips R, Sili C, Materassi R (1990) Studies on exopolysaccharide release by diazotrophic batch cultures ofCyanospira capsulata. Appl Microbiol Biotechnol 34: 392–396
Wang AW, Hill A (1977) Chemical analysis of the phenol-waterextractable materials fromAnabaena flos-aquae. J Bacteriol 130: 558–560
Weckesser J, Hofmann K, Jürgens UJ, Whitton BA, Raffelsberger B (1988) Isolation and chemical analysis of the sheaths of filamentous cyanobacteriaCalothrix parietina andC. scopulorum. J Gen Microbiol 134: 629–634
Weijmann ACM, Meuzelaar HLC (1979) Biochemical contributions to the taxonomic status of the Endogonaceae. Can J Bot 57: 284–291
Werner D (1992) TheRhizobium/Bradyrhizobium-Fabales symbiosis. In: Werner D (ed) Symbioses of plants and microbes. Chapmann and Hall, London, pp 49–167
Wettstein F v (1915)Geosiphon Fr. Wettst., eine neue, interessante Siphonee. Österr Bot Z 65: 145–156
Author information
Authors and Affiliations
Additional information
Dedicated to Professor Dr. Peter Sitte at the occasion of his 65th birthday
Rights and permissions
About this article
Cite this article
Schüßler, A., Bonfante, P., Schnepf, E. et al. Characterization of theGeosiphon pyriforme symbiosome by affinity techniques: confocal laser scanning microscopy (CLSM) and electron microscopy. Protoplasma 190, 53–67 (1996). https://doi.org/10.1007/BF01281194
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01281194