Summary
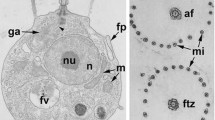
A correlated immunofluorescence and ultrastructural study of the microtubular cytoskeleton has been made in zoospores and young cysts ofPhytophthora cinnamomi. Labelling of microtubules using antibodies directed towards tubulin has revealed new details of the arrangement of the flagellar rootlets in these cells, and of the variability that occurs from cell to cell. Most of the variation exists at the distal ends of the rootlets, and may be correlated with differences in cell shape in these regions. The rootlets have the same right and left configuration in all zoospores. The arrangement of the rootlet microtubules at the anterior end of the zoospores raises the possibility that the microtubules on the left hand side of the groove may not comprise an independent rootlet which arises at the basal bodies.
The absolute configuration of the flagellar apparatus has been determined from ultrastructural observations of serial sections. In the vicinity of the basal bodies, there is little, if any, variation between individuals, and the structure of the flagellar apparatus is similar to that described for related species of fungi. Two ribbon-like coils surround the central pair of microtubules at the distal tip of the whiplash flagellum, and clusters of intramembranous particles, similar to ciliary plaques, have been found at the bases of both flagella. There are two arrays of microtubules associated with the nucleus in the zoospores. One array lies next to the outer surface of the nuclear envelope, and probably functions in the shaping and positioning of the apex of the nucleus. The nuclear pores in this region are aligned in rows alongside these microtubules. The second array is formed by kinetochore microtubules which extend into a collar-like arrangement of chromatin material around the narrow end of the (interphase) nucleus. During encystment, all flagellar rootlets are internalized when the flagella are detached at the terminal plate. The rootlets arrays are no longer recognizable 5–10 minutes after the commencement of encystment.
Similar content being viewed by others
References
Andersen RA (1985) The flagellar apparatus of the golden algaSynura uvella: four absolute orientations. Protoplasma 128: 94–106
Barr DJS (1981) The phylogenetic and taxonomic implications of flagellar rootlet morphology among zoosporic fungi. BioSystems 14: 359–370
— (1983) The zoosporic grouping of plant pathogens. Entity or nonentity? In:Buczacki ST (ed) Zoosporic plant pathogens. A modern perspective. Academic Press, London, pp 43–83
—,Allan PME (1982) Zoospore ultrastructure ofPolymyxa graminis (Plasmodiophoromycetes). Can J Bot 60: 2496–2504
— — (1985) A comparison of the flagellar apparatus inPhytophthora, Saprolegnia, Thraustochytrium, andRhizidiomyces. Can J Bot 63: 138–154
—,Hadland-Hartmann VE (1978) The flagellar apparatus in theChytridiales. Can J Bot 56: 887–900
Bernhard W (1969) A new staining procedure for electron microscopical cytology. J Ultrastruct Res 27: 250–265
Blose SH, Meltzer DI, Feramisco JR (1982) 10nm filaments induced to collapse in cells micro-injected with antibodies against tubulin. J Cell Biol 95: 229 a
Bouck GB (1975) Localization of flagellar surface growth using immunologically labeled mastigonemes as markers. In:Duckett JG, Racey PA (eds) Biology of the male gamete. Academic Press, New York, pp 15–22
Brasier CM, Sansome E (1975) Diploidy and gametangial meiosis inPhytophthora cinnamomi, P. infestans andP. dreschleri. Trans Br Mycol Soc 65: 49–65
Carter JV, Wick SM (1984) Irreversible microtubule depolymerization associated with freezing injury inAllium cepa root tip cells. Cryo Lett 5: 373–382
Cavalier-Smith T (1982) The evolutionary origin and phylogeny of eukaryote flagella. In:Amos WB, Duckett JG (eds) Prokaryotic and eukaryotic flagella. Cambridge University Press, Cambridge, pp 465–493
Dentler WL (1981) Microtubule-membrane interactions in cilia and flagella. Int Rev Cytol 72: 1–47
Galway ME, Hardham AR (1986) Microtubule reorganization, cell wall synthesis and establishment of the axis of elongation in regenerating protoplasts of the algaMougeotia. Protoplasma 135: 130–143
Giloh H, Sedat JW (1982) Fluorescence microscopy: reduced photobleaching of rhodamine and fluorescein protein conjugates by n-propyl gallate. Science 217: 1252–1255
Gorst J, Wernicke W, Gunning BES (1986) Is the preprophase band of microtubules a marker of organization in suspension cultures? Protoplasma 134: 130–140
Gotelli D (1974) The morphology ofLagenidium callinectes. II. Zoosporogenesis. Mycologia 66: 846–858
Gunning BES, Steer MW (1975) Ultrastructure and the biology of plant cells. Edward Arnold, London, pp 312
—,Wick SM (1985) Preprophase bands, phragmoplasts, and spatial control of cytokinesis. J Cell Sci [Suppl] 2: 157–179
Hardham AR (1985) Studies on the cell surface of zoospores and cysts of the fungusPhytophthora cinnamomi: the influence of fixation on patterns of lectin binding. J Histochem Cytochem 33: 110–118
—,Suzaki E (1986) Encystment of zoospores of the fungus,Phytophthora cinnamomi, is induced by specific lectin and monoclonal antibody binding to the cell surface. Protoplasma 133: 165–173
— —,Perkin JL (1986) Monoclonal antibodies to isolate-, species- and genus-specific components on the surface of zoospores and cysts of the fungusPhytophthora cinnamomi. Can J Bot 64: 311–321
Heath IB, Greenwood AD (1971) Ultrastructural observations on the kinetosomes, and Golgi bodies during the asexual life cycle ofSaprolegnia. Z Zellforsch 112: 371–389
—,Rethoret K (1982) Mitosis in the fungusZygorhynchus moelleri: Evidence for stage specific enhancement of microtubule preservation by freeze substitution. Eur J Cell Biol 28: 180–189
Held AA (1972) Fungal zoospores are induced to encyst by treatments known to degrade cytoplasmic microtubules. Arch Mikrobiol 85: 209–224
Hepler PK (1981) The structure of the endoplasmic reticulum revealed by osmium tetroxide-potassium ferricyanide staining. Eur J Cell Biol 26: 102–110
Hoch HC, Mitchell JE (1972) The ultrastructure of zoospores ofAphanomyces euteiches and of their encystment and subsequent germination. Protoplasma 75: 113–138
—,Staples RC (1985) The microtubule cytoskeleton in hyphae ofUromyces phaseoli germlings: its relationship to the region of nucleation and to the f-actin cytoskeleton. Protoplasma 124: 112–122
Hogetsu T, Oshima Y (1985) Immunofluorescence microscopy of microtubule arrangement inClosterium acerosum (Schrank) Ehrenberg. Planta 166: 169–175
— — (1986) Immunofluorescence microscopy of microtubule arrangement in root cells ofPisum sativum L. var Alaska. Plant Cell Physiol 27: 939–945
Holloway SA, Heath IB (1977) Morphogenesis and the role of microtubules in synchronous populations ofSaprolegnia zoospores. Exp Mycol 1: 9–29
Kazama F (1980) The zoospore ofSchizochytrium aggregatum. Can J Bot 58: 2434–2446
Kilmartin JV, Wright B, Milstein C (1982) Rat monoclonal antitubulin antibodies derived by using a new nonsecreting rat cell line. J Cell Biol 93: 576–582
Lunney CZ, Bland CE (1976) Ultrastructural observations of mature and encysting zoospores ofPythium proliferum de Bary. Protoplasma 90: 119–137
Marc J, Gunning BES (1986) Immunofluorescent localization of cytoskeletal tubulin and actin during spermatogenesis inPteridium aquilinum (L.) Kuhn. Protoplasma 134: 163–177
Melkonian M, Kröger K-H, Marquardt K-G (1980) Cell shape and microtubules in zoospores of the green algaChlorosarcinopsis gelatinosa (Chlorosarcinales): effects of low temperature. Protoplasma 104: 283–293
Mizuno K, Sek F, Perkin J, Wick S, Duniec J, Gunning B (1985) Monoclonal antibodies specific to plant tubulin. Protoplasma 129: 100–108
Overton SV, Tharp TP, Bland CE (1983) Fine structure of swimming, encysting, and germinating spores ofHaliphthoros milfordensis. Can J Bot 61: 1165–1177
Paolillo DJ, Jr (1967) On the structure of the axoneme in flagella ofPolytrichum juniperinum. Trans Am Microsc Soc 86: 428–433
Plattner H (1975) Ciliary granule plaques: Membrane-intercalated particle aggregates associated with Ca2+-binding sites inParamecium. J Cell Sci 18: 257–269
Reichle RE (1969) Fine structure ofPhytophthora parasitica zoospores. Mycologia 61: 30–51
Sattler CA, Staehelin LA (1974) Ciliary membrane differentiations inTetrahymena pyriformis. J Cell Biol 62: 473–490
Schnepf E, Deichgräber G, Drebes G (1978) Development and ultrastructure of the marine, parasitic Oomycete,Lagenisma coscinodisci Drebes (Lagenidiales): formation of the primary zoospores and their release. Protoplasma 94: 263–280
— —,Röderer G, Herth W (1977) The flagellar root apparatus, the microtubular system and associated organelles in the Crysophycean flagellate,Poteriochromonas malhamensis Peterfi (syn.Poteriochromonas stipitata Scherffel andOchromonas malhamensis Pringsheim). Protoplasma 92: 87–107
Vujičić R, Colhoun J, Chapman JA (1968) Some observations on the zoospores ofPhytophthora erythroseptica. Trans Br Mycol Soc 51: 125–127
Wasteneys GO,Williamson RE (1987) Microtubule orientation in developing internodal cells ofNitella: a quantitative analysis. Eur J Cell Biol. In press
Weber K, Wehland J, Herzog W (1976) Griseofulvin interacts with microtubules bothin vivo andin vitro. J Molec Biol 102: 817–829
Wehland J, Schroeder M, Weber K (1984) Organization of microtubules in stabilized meristematic plant cells revealed by a rat monoclonal antibody reacting only with the tyrosinated form of α-tubulin. Cell Biol Int Rep 8: 147–150
Wick SM, Duniec J (1983) Immunofluorescence microscopy of tubulin and microtubule arrays in plant cells. I. Preprophase band development and concomitant appearance of nuclear envelopeassociated tubulin. J Cell Biol 97: 235–243
— — (1984) Immunofluorescence microscopy of tubulin and microtubule arrays in plant cells. II. Transition between the preprophase band and the mitotic spindle. Protoplasma 122: 45–55
— — (1986) Effects of various fixatives on the reactivity of plant tubulin and calmodulin in immunofluorescence microscopy. Protoplasma 133: 1–18
—,Muto S, Duniec J (1985) Double immunofluorescence labelling of calmodulin and tubulin in dividing plant cells. Protoplasma 126: 198–206
—,Seagull RW, Osborn M, Weber K, Gunning BES (1981) Immunofluorescence microscopy of organized microtubule arrays in structurally stabilized meristematic plant cells. J Cell Biol 89: 685–690
Williams WT, Webster RK (1970) Electron microscopy of the sporangium ofPhytophthora capsici. Can J Bot 48: 221–227
Zentmyer GA (1980)Phytophthora cinnamomi and the diseases it causes. The American Phytopathological Society, St Paul, p 96
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hardham, A.R. Microtubules and the flagellar apparatus in zoospores and cysts of the fungusPhytophthora cinnamomi . Protoplasma 137, 109–124 (1987). https://doi.org/10.1007/BF01281146
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01281146