Summary
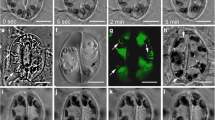
Stomatal-pore formation in the fern Asplenium nidus L. commences in postcytokinetic guard cells at the mid-region of the ventral wall, before the deposition of any cellulosic wall material on it, by the local movement of the adjacent plasmalemmata apart from each other. In this way a rudimentary “internal stomatal pore” is formed. At this stage the ventral wall exhibits an undulated appearance and gives a positive reaction to aniline blue. Detailed study of postcytokinetic guard cells by electron microscopy, as well as after tubulin immunolabeling and actin staining, shows that stomatal pore initiation coincides with the initiation of the organization of the anticlinal microtubule bundles along the middle of the ventral wall and the colocalization of actin filaments at the same sites. Afterwards, the stomatal pore broadens towards the periclinal walls, a phenomenon keeping pace with the further bundling of the cytoskeletal elements beneath the plasmalemmata lining the middle of the ventral wall. At this stage the anticlinal microtubule bundles lining the stomatal pore are very prominent. The above findings, as well as the fact that treatments with antimicrotubule drugs inhibit the “internal” stomatal-pore formation, denote that the cortical cytoskeleton lining the ventral wall and particularly the microtubules are involved in this process. Afterwards, distinct local wall thickenings are deposited at the sites of junction of the mid-region of the ventral wall with the periclinal walls as well as at the junctions of the polar ventral-wall ends with the external periclinal wall. Along the middle-lamella region of the former wall thickenings the fore- and rear-chambers of the stomatal pore are formed. The final stomatal-pore opening is achieved by disruption of the expanded thin median periclinal wall region inherited from the guard cell mother cell and of the overlying cuticle, which covers the stomatal pore externally and internally. At the same time the fore-chamber of the stomatal pore broadens by a schizogenous opening towards the polar ventral-wall ends. The observations show that the stomatal-pore formation in A. nidus is a unique process, which is probably restricted to ferns.
Similar content being viewed by others
Abbreviations
- Af:
-
actin filament
- GC:
-
guard cell
- Mt:
-
microtubule
- MSB:
-
microtubule-stabilizing buffer
- PBS:
-
phosphate-buffered saline
- VW:
-
ventral wall
References
Apostolakos P, Panteris E, Galatis B (1997) Microtubule and actin filament organization during stomatal morphogenesis in the fern Asplenium nidus I: guard cell mother cell. Protoplasma 198: 93–106
Busby CH, Gunning BES (1984) Microtubules and morphogenesis in stomata of the water fern Azolla: an unusual mode of guard cell and pore development. Protoplasma 22: 108–119
Cleary AL, Hardham AR (1989) Microtubule organization during development of stomatal complexes in Lolium rigidum. Protoplasma 149: 67–81
— — (1990) Reinstatement of microtubule arrays from cortical nucleating sites in stomatal complexes of Lolium rigidum following depolymerization of microtubules by oryzalin and high pressure. Plant Cell Physiol 31: 903–915
Galatis B (1980) Microtubules and guard-cell morphogenesis in Zea mays L. J Cell Sci 45: 211–244
—, Apostolakos P (1991) Microtubule organization and morphogenesis of stomata in caffeine-affected seedlings of Zea mays. Protoplasma 165: 11–26
—, Mitrakos K (1980) The ultrastructural cytology of the differentiating guard cells of Vigna sinensis. Am J Bot 67: 1243–1261
—, Apostolakos P, Katsaros C (1983) Microtubules and their organizing centres in differentiating guard cells of Adiantum capillus-veneris. Protoplasma 115: 176–192
Goto Y, Ueda K (1988) Microfilament bundles of F-actin in Spirogyra observed with fluorescence microscopy. Planta 173: 442–446
Lokhorst GM, Star W (1983) Fine structure of mitosis and cytokinesis in Urospora (Acrosiphoniales, Chlorophyta). Protoplasma 117: 142–153
— — (1985) Ultrastructure of mitosis and cytokinesis in Klebsormidium mucosum Nov. Comb., formerly Ulothrix verrucosa (Chlorophyta). J Phycol 21: 466–476
Meindl U (1986) Autonomous circular and radial motions of the nucleus in Pleurenterium tumidum and their relation to cytoskeletal elements and the plasma membrane. Protoplasma 135: 50–66
O'Brien TP, McCully ME (1981) The study of plant structure: principles and selected methods. Termarcarphi Pty, Melbourne
Panteris E, Galatis B, Apostolakos P (1991) Patterns of cortical and perinuclear microtubule organization in meristematic root cells of Adiantum capillus-veneris. Protoplasma 165: 173–188
—, Apostolakos P, Galatis B (1992) The organization of F-actin in root tip cells of Adiantum capillus-veneris throughout the cell cycle: a double label fluorescence microscopy study. Protoplasma 170: 128–137
— — — (1993) Microtubule organization, mesophyll cell morphogenesis and intercellular space formation in Adiantum capillus-veneris leaflets. Protoplasma 172: 97–110
Peterson RL, Hambleton S (1978) Guard cell ontogeny in leaf stomata of the fern Ophioglossum petiolatum. Can J Bot 56: 2836–2852
—, Firminger MS, Dobrindt LA (1975) Nature of the guard cell wall in leaf stomata of three Ophioglossum species. Can J Bot 53: 1698–1711
Sack FD (1987) The development and structure of stomata. In: Zeiger E, Farquhar GD, Cowan IR (eds) Stomatal function. Stanford University Press, Stanford, pp 59–89
—, Paolillo DJ Jr (1983) Stomatal pore and cuticle formation in Funaria. Protoplasma 116: 1–13
Stevens RA, Martin ES (1978) Structural and functional aspects of stomata I: developmental studies in Polypodium vulgare. Planta 142: 307–316
Zachariadis M, Apostolakos P, Galatis B (1997) Morphogenesis of “floating” stomata in the fern Anemia mandioccana: stomatal-pore formation. In: Abstracts of First Balkan Botanical Congress, Thessaloniki, September 19–22, 1997, p 372
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Apostolakos, P., Galatis, B. Probable involvement of cytoskeleton in stomatal-pore formation in Asplenium nidus L.. Protoplasma 203, 48–57 (1998). https://doi.org/10.1007/BF01280586
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01280586