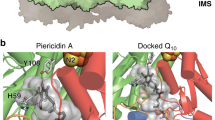
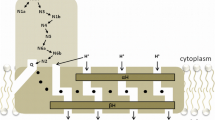
Summary
The NADH:ubiquinone oxidoreductase (complex I) of the mitochondrial respiratory chain is by far the most complicated of the proton-translocating enzymes involved in the oxidative phosphorylation. Many clues regarding both electron transfer and proton translocation are still unknown. In this sense, inhibitor assays are relevant and useful pieces for elaborating a suitable model to explain the elusive bioenergetic mechanism of this enzyme. This short review presents the most recent advances in inhibitor studies and highlights the major controversies.
Similar content being viewed by others
Abbreviations
- ACG:
-
annonaceous acetogenin
- MPP+ :
-
methylphenyl-pyridinium
References
Alali FQ, Liu XX, McLaughlin JL (1999) Annonaceous acetogenins: recent progress. J Nat Prod 62: 504–540
Brandt U (1997) Proton-translocation by membrane-bound NADH:ubiquinone-oxidoreductase (complex I) through redoxgated ligand conduction. Biochim Biophys Acta 1318: 79–91
— (1999) Proton-translocation in the respiratory chain involving ubiquinone: a hypothetical semiquinone switch mechanism for complex I. Biofactors 9: 95–101
Carelli V, Ghelli A, Ratta M, Bacchilega E, Sangiorgi S, Mancini R, Leuzzi V, Cortelli P, Montagna P, Lugaresi E, Degli Esposti M (1997) Leber's hereditary optic neuropathy: biochemical effect of 11778/ND4 and 3460/ND1 mutations and correlation with the mitochondrial genotype. Neurology 48: 1623–1632
Darrouzet E, Dupuis A (1997) Genetic evidence for the existence of two quinone related inhibitor binding sites in NADH-CoQ reductase. Biochim Biophys Acta 1319: 1–4
Degli Esposti M (1998) Inhibitors of NADH-ubiquinone reductase: an overview. Biochim Biophys Acta 1364: 222–235
—, Ghelli A (1994) The mechanism of proton and electron transport in mitochondrial complex I. Biochim Biophys Acta 1187: 116–120
— — (1999) Ubiquinone and inhibitor binding sites in complex I: one, two or three? Biochem Soc Trans 27: 606–609
— —, Crimi M, Estornell E, Fato R, Lenaz G (1993) Complex I and complex III of mitochondria have common inhibitors acting as ubiquinone antagonists. Biochem Biophys Res Commun 190: 1090–1096
— —, Ratta M, Cortes D, Estornell E (1994a) Natural substances (acetogenins) from the family Annonaceae are powerful inhibitors of mitochondrial NADH dehydrogenase (complex I). Biochem J 301: 161–167
—, Crimi M, Ghelli A (1994b) Natural variation in the potency and binding sites of mitochondrial quinone-like inhibitors. Biochem Soc Trans 22: 209–213
—, Ngo A, McMullen GL, Ghelli A, Sparla F, Benelli B, Ratta M, Linnane AW (1996) The specificity of mitochondrial complex I for ubiquinones. Biochem J 313: 327–334
Dutton PL, Moser CC, Sled VD, Daldal F, Ohnishi T (1998) A reductant-induced oxidation mechanism for complex I. Biochim Biophys Acta 1364: 245–257
Estornell E, Fato R, Pallotti F, Lenaz G (1993) Assay conditions for the mitochondrial NADH:coenzyme Q oxidoreductase. FEBS Lett 332: 127–131
—, Tormo JR, Barber T (1997a) A deficiency in respiratory complex I in heart mitochondria from vitamin A-deficient rats is counter-acted by an increase in coenzyme Q. Biochem Biophys Res Commun 233: 451–454
— —, Cortes D (1997b) Cherimolin-1, new selective inhibitor of the first energy-coupling site of the NADH:ubiquinone oxidoreductase (complex I). Biochem Biophys Res Commun 240: 234–238
Fato R, Estornell E, Di Bernardo S, Pallotti F, Parenti-Castelli G, Lenaz G (1996) Steady-state kinetics of the reduction of coenzyme Q analogs by complex I (NADH:ubiquinone oxidoreductase) in bovine heart mitochondria and submitochondrial particles. Biochemistry 35: 2705–2716
Friedrich T, Van Heek P, Leif H, Ohnishi T, Forche E, Kunze B, Jansen R, Trowitzsch-Kienast W, Höfle G, Reichenbach H, Weiss H (1994) Two binding sites of inhibitors in NADH: ubiquinone oxidoreductase (complex I). Eur J Biochem 219: 691–698
Gluck MR, Krueger MJ, Ramsay RR, Sablin SO, Singer TP, Nicklas WJ (1994) Characterization of the inhibitory mechanism of 1-methyl-4-phenylpyridinium and 4-phenylpyridine analogs in inner membrane preparations. J Biol Chem 269: 3167–3174
González MC, Tormo JR, Bermejo A, Zafra-Polo MC, Estornell E, Cortes D (1997) Rollimembrin, a novel acetogenin inhibitor of mammalian mitochondrial complex I. Bioorg Med Chem Lett 7: 1113–1118
Helfenbaum L, Ngo A, Ghelli A, Linnane AW, Degli Esposti M (1997) Proton pumping of mitochondrial complex I: differential activation by analogs of ubiquinone. J Bioenerg Biomembr 29: 71–80
Iwata J, Miyoshi H, Iwamura H (1999) Origin of selective inhibition of mitochondrial complex I by pyridinium-type inhibitor MP-24. Biochim Biophys Acta 1413: 63–69
Lenaz G (1998) Quinone specificity of complex I. Biochim Biophys Acta 1364: 207–221
Lümmen P (1998) Complex I inhibitors as insecticides and acaricides. Biochim Biophys Acta 1364: 287–296
— (1999) Biochemical aspects of N-heterocyclic complex-I inhibitors with insecticidal activity. Biochem Soc Trans 27: 602–606
Miyoshi H (1998) Structure-activity relationships of some complex I inhibitors. Biochim Biophys Acta 1364: 236–244
—, Inoue M, Okamoto S, Ohshima M, Sakamoto K, Iwamura H (1997) Probing the ubiquinone reduction site of mitochondrial complex I using novel cationic inhibitors. J Biol Chem 272: 16176–16183
—, Iwata J, Sakamoto K, Furukawa H, Takada M, Iwamura H, Watanabe T, Kodama Y (1998a) Specificity of pyridinium inhibitors of the quinone reduction sites in mitochondrial complex I. J Biol Chem 273: 17368–17274
—, Ohshima M, Shimada H, Akagi T, Iwamura H, McLaughlin JL (1998b) Essential structural factor of Annonaceous acetogenins as potent inhibitors of mitochondrial complex I. Biochim Biophys Acta 1365: 443–452
Oberlies NH, Jones IL, Corbett TH, Fotopoulos SS, McLaughlin JL (1995) Tumor cell growth inhibition by several Annonaceous acetogenins in an in vitro disk diffusion assay. Cancer Lett 96: 55–62
—, Chang C, McLaughlin JL (1997) Structure-activity relationships of diverse Annonaceous acetogenins against multidrug-resistant human mammary adenocarcinoma (MCF-7/Adr) cells. J Med Chem 40: 2102–2106
Ohnishi T (1998) Iron-sulfur clusters: semiquinones in complex I. Biochim Biophys Acta 1364: 186–206
—, Magnitsky S, Toulokhonova L, Yano T, Yagi T, Burbaev DS, Vinogradov AD (1999) EPR studies of the possible binding sites of the cluster N2, semiquinones, and specific inhibitors of the NADH:ubiquinone oxidoreductase (complex I). Biochem Soc Trans 27: 586–591
Ohshima M, Miyoshi H, Sakamoto K, Takegami K, Iwata J, Kuwabara K, Iwamura H, Yagi T (1998) Characterization of the ubiquinone reduction site of mitochondrial complex I using bulky synthetic ubiquinones. Biochemistry 37: 6436–6445
Okun JG, Lümmen P, Brandt U (1999a) Three classes of inhibitors share a common binding domain in mitochondrial complex I (NADH:ubiquinone oxidoreductase). J Biol Chem 274: 2625–2630
—, Zickermann V, Brandt U (1999b) Properties of the common inhibitor-binding domain in mitochondrial NADH-dehydrogenase (complex I). Biochem Soc Trans 27: 596–601
Reil E, Höfle G, Draber W, Oettmeier W (1997) Quinolones and their N-oxides as inhibitors of mitochondrial complexes I and III. Biochim Biophys Acta 1318: 291–298
Robinson BH (1998) Human complex I deficiency: clinical spectrum and involvement of oxygen free radicals in the pathogenicity of the defect. Biochim Biophys Acta 1364: 271–286
Schapira AHV (1998) Human complex I defects in neurodegenerative diseases. Biochim Biophys Acta 1364: 261–270
Schuler F, Yano T, Di Bernardo S, Yagi T, Yankovskaya V, Singer TP, Casida JE (1999) NADH-quinone oxidoreductase: PSST subunit couples electron transfer from iron-sulfur cluster N2 to quinone. Proc Natl Acad Sci USA 96: 4149–4153
Suomalainen A (1997) Mitochondrial DNA and disease. Ann Med 29: 235–246
Tormo JR, González MC, Cortes D, Estornell E (1999a) Kinetic characterization of mitochondrial complex I inhibitors using Annonaceous acetogenins. Arch Biochem Biophys 369: 119–126
—, Gallardo T, Aragón R, Cortes D, Estornell E (1999b) Specific interactions of monotetrahydrofuranic Annonaceous acetogenins as inhibitors of mitochondrial complex I. Chem Biol Interact 122: 171–183
Ueno H, Miyoshi H, Inoue M, Niidome Y, Iwamura H (1996) Structural factors of rotenone required for inhibition of various NADH-ubiquinone oxidoreductases. Biochim Biophys Acta 1276: 195–202
Ushakiva A, Grivennikova VG, Ohnishi T, Vinogradov AD (1999) Triton X-100 as a specific inhibitor of the mammalian NADH: ubiquinone oxidoreductase (complex I). Biochim Biophys Acta 1409: 143–153
Vinogradov AD (1998) Catalytic properties of the mitochondrial NADH-ubiquinone oxidoreductase (Complex I) and the pseudoreversible active/inactive enzyme transition. Biochim Biophys Acta 1364: 169–185
Wallace DC (1992) Diseases of the mitochondrial DNA. Annu Rev Biochem 61: 1175–1212
— (1999) Mitochondrial diseases in man and mouse. Science 283: 1482–1488
Zafra-Polo MC, González MC, Tormo JR, Estornell E, Cortes D (1996) Polyalthidin: new prenylated benzopyran inhibitor of the mammalian mitochondrial respiratory chain. J Nat Prod 59: 913–916
—, Figadère B, Gallardo T, Tormo JR, Cortes D (1998) Natural acetogenins from Annonaceae, synthesis and mechanism of action. Phytochemistry 48: 1087–1117
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Estornell, E. Mitochondrial complex I: new insights from inhibitor assays. Protoplasma 213, 11–17 (2000). https://doi.org/10.1007/BF01280500
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01280500