Summary
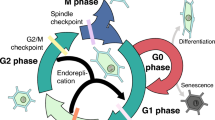
The cell cycle of an arbuscular mycorrhizal fungus,Glomus versiforme, was determined by flow cytometric analysis of nuclei isolated from spores and mycorrhizal roots of leek, and by immunogold staining after bromodeoxyuridine (BrdU) uptake by DNA. The aims of our work were to establish: (i) whether there are changes in ploidy during fungal growth and morphogenesis, (ii) when and where the cell cycle is activated. Our results demonstrate that nuclei isolated from quiescent spores ofG. versiforme are arrested in the GO/G1 phase (99.2%), whereas fungal nuclei from mycorrhizal roots are in the synthetic (S) (10.1%) and G2/M phase (3.9%). Nuclei undergoing DNA synthesis were detected in situ after BrdU uptake. Labelled nuclei were observed in intercellular hyphae and in large arbuscular trunks. This paper demonstrates that colonization of an arbuscular mycorrhizal fungus is linked to activation of its cell cycle.
Similar content being viewed by others
Abbreviations
- AM:
-
fungi arbuscular mycorrhizal fungi
- BrdU:
-
5-bromo-2′-deoxyuridine
- PI:
-
propidium iodide
- DAPI:
-
4′,6-diamidino-2-phenylindole
References
Balestrini R, Bianciotto V, Bonfante P (1992) Nuclear architecture and DNA location in two VAM fungi. Mycorrhiza 1: 105–112
Barbiero G, Duranti F, Bonelli G, Amenta JS, Baccino FM (1995) Intracellular ionic variations in the apoptotic death of L cells by inhibitors of cell cycle progression. Exp Cell Res 217: 410–418
Becard G, Fortin JA (1988) Early events of vesicular-arbuscular mycorrhiza formation on Ri-TDNA transformed roots. New Phytol 108: 211–218
—, Pfeffer PE (1993) Status of nuclear division in arbuscular mycorrhizal fungi during in vitro development. Protoplasma 174: 62–68
—, Piché Y (1989) New aspects on the acquisition of biotrophic status by a vesicular-arbuscular mycorrhizal fungusGigaspora margarita. New Phytol 112: 77–83
—, Douds DD, Pfeffer PE (1992) Extensive in vitro hyphal growth of vesicular-arbuscular mycorrhizal fungi in the presence of CO2 and flavonols. Appl Environ Microbiol 58: 821–825
Berta G, Sgorbati S, Soleri V, Fusconi A, Trotta A, Citterio S, Sparvoli E, Scannerini S (1990) Variations in chromatin structure in host nuclei of a vesicular arbuscular mycorrhiza. New Phytol 114: 199–205
Bianciotto V, Bonfante P (1992) Quantification of the nuclear DNA content of two arbuscular mycorrhizal fungi. Mycol Res 96: 1071–1076
— — (1993) Evidence of DNA replication in an arbuscular mycorrhizal fungus in the absence of the host plant. Protoplasma 176: 100–105
Bonfante P (1984) Anatomy and morphology of VA mycorrhizae. In: Powell CL, Bagyaraj DJ (eds) VA mycorrhizas. CRC Press, Boca Raton, pp 5–33
—, Perotto S (1992) Plants and endomycorrhizal fungi: the cellular and molecular basis of their interaction. In: Verma DPS (ed) Molecular signals in plant-microbe communications. CRC Press, Boca Raton, pp 445–470
—, Balestrini R, Mendgen K (1994) Storage and secretion processes in the spore ofGigaspora margarita Becker & Hall as revealed by high-pressure freezing and freeze substitution. New Phytol 128: 93–101
Brundrett MC, Piché Y, Peterson RL (1985) A developmental study of the early stages in vesicular mycorrhiza formation. Can J Bot 63: 184–194
Darzynkiewicz Z, Crissman HA (1991) Methods in cell biology, vol 33, flow cytometry. Academic Press, San Diego
De Rocher EJ, Harkins KR, Galbraith DW, Bohnert HJ (1990) Developmentally regulated systemic endopolyploidy in succulents with small genomes. Science 250: 99–101
Dien SB, Scrienc F (1991) Bromodeoxyuridine labelling and flow cytometric identification of replicatingSaccharomyces cerevisiae cells: lengths of cell cycle phases and population variability at specific cell cycle positions. Biotechnol Prog 7: 291–298
Francis D (1992) The cell cycle in plant development. New Phytol 122: 1–20
Galbraith DW, Harkins KR, Maddox JM, Ayres NM, Sharma DP, Firoozabady E (1983) Rapid flow cytometric analysis of the cell cycle in intact plant tissue. Science 220: 1049–1051
Georgieva EI, López-Rodas G, Hittmair A, Feichtinger H, Broch G, Loidl P (1994) Maize embryo germination. I. Cell cycle analysis. Planta 192: 118–124
Gianinazzi-Pearson V, Branzanti B, Gianinazzi S (1989) In vitro enhancement of spore germination and early hyphal growth of a vesicular-arbuscular mycorrhizal fungi by host root exudates and plant flavonoids. Symbiosis 7: 243–255
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 84: 489–500
—, Avio L, Sbrana C, Citernesi AS (1993) Factors affecting appressorium development in the vesicular-arbuscular mycorrhizal fungusGlomus mosseae (Nicol. & Gerd.) Gerd. & Trappe. New Phytol 123: 115–122
Harley JL (1989) The significance of mycorrhiza. Mycol Res 92: 92–129
Haugland RP (1993) Handbook of fluorescent probes and research chemicals, 5th edn. Molecular Probes, Eugene, OR
Hewitt EJ (1966) Sand and water culture methods used in the studies of plant nutrition. Commonwealth Agricultural Bureau, London, Tech Commun 22
Hill DJ (1989) The control of the cell cycle in microbial symbionts. New Phytol 112: 175–184
Hosny M, Dulieu H (1994) Organization of the genome ofScutellospora castanea, an endomycorrhizal fungus. In: Fourth European Symposium on Mycorrhizas, Granada, 11–14 July 1994, p 90
Oinuma T, Kawano J, Suganuma T (1992) Bromodeoxyuridine-immunohistochemistry on cellular differentiation and migration in the fundic gland ofXenopus laevis during development. Cell Tissue Res 269: 205–212
Peters NK, Verma DPS (1990) Phenolic compounds as regulators of gene expression in plant-microbe interactions. Mol Plant Microbe Interact 3: 4–8
Simon L, Bousquet J, Lévesque RC, Lalonde M (1993) Origin and diversification of endomycorrhizal fungi and coincidence with vascular land plants. Nature 363: 67–68
Smith DC (1993) The symbiotic condition. Symbiosis 14: 3–15
Smith SE, Gianinazzi-Pearson V, Koide R, Cairney JWG (1994) Nutrient transport in mycorrhizas: structure, physiology and consequences for efficiency of the symbiosis. In: Robson AD, Abbott LK, Malajczuk N (eds) Management of mycorrhizas in agriculture, horticulture and forestry. Kluwer, Dodrecht, pp 103–113
—, Gianinazzi-Pearson V (1988) Physiological interactions between symbionts in vesicular mycorrhizal plants. Annu Rev Plant Physiol Mol Biol 39: 221–244
Stroobants C, Sossountzoz L, Miginiac E (1990) DNA synthesis in excised tobacco leaves after bromodeoxyuridine incorporation: immunohistochemical detection in semi-thin Spurr sections. J Hist Cytol 38: 641–647
Wangemann-Budde M, Schauz K (1991) Intraspecific hybridization ofUstilago maydis haploids with compatible and incompatible mating type by electrofusion and genetic analysis of the fusion products. Exp Mycol 15: 159–166
Ylstra B, Touraev A, Benito Moreno RM, Stroger E, Van Tunen AJ, Vicente O, Mol JNM, Heberle-Bors E (1992) Flavonols stimulate development, germination, and tube growth of tobacco pollen. Plant Physiol 100: 902–907
Zézé A, Dulieu H, Gianinazzi-Pearson V (1994) DNA cloning and screening of a partial genomic library from an arbuscular mycorrhizal fungus,Scutellospora castanea. Mycorrhiza 4: 251–254
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bianciotto, V., Barbiero, G. & Bonfante, P. Analysis of the cell cycle in an arbuscular mycorrhizal fungus by flow cytometry and bromodeoxyuridine labelling. Protoplasma 188, 161–169 (1995). https://doi.org/10.1007/BF01280367
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01280367