Summary
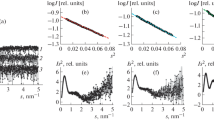
Small angle X-ray scattering measurements and electron microscopic studies were carried out onE. coli phosphofructokinase (E.C. 2.7.1.11; ATP: D-fructose-6-phosphate-1-phosphotransferase). The results suggest a tetrahedral arrangement of the protomers resulting in a radius of gyration of the enzyme of R=34.6 Å and a Stokes' radius of R0=44.0 Å. The stereochemical arrangement of the four protomers, each of a molecular weight of 35,000, within theE. coli enzyme was further substantiated by a comparison of theoretical scattering functions with the experimental scattering measurements in dilute solutions of phosphofructokinase under physiological conditions. Moreover, from other hydrodynamic measurements,e.g., intrinsic viscosity and sedimentation coefficient, theMandelkern-Scheraga factor, β, was calculated to be 2,095×106, which is significantly lower than the β0 for rigid spheres of 2,112×106. This low β-value might be due to a considerable porosity of the four protomers for mobile water molecules. The β-value of 2,095×106 is an indication of a porous sphere of almost uniform density at aDebye shielding ratio of 6.5, corresponding to a sphere radius of 22.0 Å for one protomer and an inverse hydrodynamic shielding length of 0.45 Å−1.
Similar content being viewed by others
References
Aaronson, R. P., andC. Frieden, 1972: Rabbit muscle phosphofructokinase: Studies on the polymerisation. The behavior of the enzyme at pH 8, pH 6 and intermediate pH values. J. biol. Chem.247, 7502–7504.
Blangy, D., H. Buc, andJ. Monod, 1968: Kinetics of the allosteric interaction of phosphofructokinase fromE. coli. J. molec. Biol.31, 13–35.
—, 1968: Phosphofructokinase fromE. coli.: Evidence for a tetrameric structure of the enzyme. FEBS-Letters2, 109–111.
Bock, P. E., andC. Frieden, 1976 a: Phosphofructokinase; I. Mechanism of the pHdependent inactivation and reactivation of the rabbit muscle enzyme. J. biol. Chem.251, 5630–5636.
—, andE. Frieden, 1976 b: Phosphofructokinase; II. Role of ligands in pH-dependent structural changes of the rabbit muscle enzyme. J. biol. Chem.251, 5637–5643.
—, andC. Frieden, 1976 c: Phosphofructokinase; III. Correlation of the regulatory kinetic and molecular properties of the rabbit muscle enzyme. J. biol. Chem.251, 5644–5647.
Brand, I. A., M. K. Müller, C. Unger, andH. D. Sölling, 1976:In vivo andin vitro interconversions of active and inactive forms of phosphofructokinase in rat liver. FEBSLetters68, 271–274.
Cass, K. H., andE. Stellwagen, 1975: A thermostable phosphofructokinase from the extreme thermophile Thermus X-1. Arch. Biochem. Biophys.171, 682–694.
Debye, P., 1915: Zerstreuung von Röntgenstrahlen. Ann. Physik46, 809–823.
—, 1930: Röntgeninterferenzen und Atomgröße. Physik. Z.31, 419–428.
—, andA. M. Bueche, 1953: Intrinsic viscosity, defusion and sedimentation rate of polymers insolution. J. amer. chem. Soc.75, 179–194.
Guinier, A., 1939: La diffraction des sayons X aux tres petit angles: application àl'étude de phenomen ultramicroscopiques. Ann. Phys.12, 161–237.
—, andG. Fournet, 1955: In: Small-angle Scattering of X-rays. New York, Inc.
Kahovec, L., G. Porod, andH. Ruck, 1953: Röntgenkleinwinkeluntersuchungen an dicht gepackten kolloiden Systemen. Kolloid-Z.133, 16–26.
Kono, N., K. Uyeda, andR. M. Oliver, 1973: Chicken liver phosphofructokinase. I. Crystallization and physico-chemical properties. J. biol. Chem.248, 8592–8602.
— —, 1973: Chicken liver phosphofructokinase. II. Cold inactivation. J. biol. Chem.243, 8603–8609.
Kauzmann, W., K. Moore, andD. Schultz, 1974: Protein densities from X-ray crystallographic coordinates. Nature248, 447–449.
Koshland, D. E., G. Nemethy, andD. Filmer, 1966: Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry5, 365–385.
Lad, P. M., andG. G. Hammes, 1974: Physical and chemical properties of rabbit muscle phosphofructokinase cross-linked with dimethylsuberimidate. Biochemistry13, 4530–4537.
—,D. E. Hill, andG. G. Hammes, 1973: Influence of allosteric ligands on the activity and aggregation of rabbit muscle phosphofructokinase. Biochemistry12, 4303–4309.
Lake, J. A., 1967: An interative method of slit correcting small-angle X-ray data. Acta Cryst.23, 191–194.
Mansour, T. E., 1972: Phosphofructokinase. In: Current topics in cellular regulation (Horecker, B. L., E. R. Stadtman, eds.), Vol. 5, pp. 1–46. New York-London: Acacemic Press.
Marschke, Ch., andR. W. Bernlohr, 1973: Purification and characterization of phosphofructokinase ofBacillus licheniformis. Arch. Biochem. Biophys.156, 1–16.
Monod, J., J. P. Changeux, andFr. Jacob, 1963: Allosteric proteins and cellular control system. J. molec. Biol.6, 306–329.
Paradies, H. H., andA. Franz, 1976: Geometry of the protein S 4 fromE. coli. ribosomes. Eur. J. Biochem.67, 23–30.
—, 1971: Polymorphism of serine specific transfer ribonucleic acid. Eur. J. Biochem.18, 530–540.
—, andW. Vettermann, 1976 b: On the quaternary structure of native rabbit muscle phosphofructokinase. Biochem. biophys. Res. Comm.71, 520–526.
—,B. Zimmer, andG. Werz, 1976 a: Small-angle X-ray scattering of D-ribulose-1,5-biphosphate carboxylase fromDasycladus clavaeformis ROTH (Ag.) in solution. Biochem. biophys. Res. Comm.74, 397–404.
- and W.Vettermann, 1977: A physical model of the polymers of phosphofructokinase from rabbit muscle. Submitted to Proc. nat. Acad. Sci. (U.S.A.).
Porod, G., 1951: Die Röntgenkleinwinkelstreuung von dicht gepackten kolloiden Systemen, I. Teil: Kolloid-Z.124, 73–114. II. Teil: Kolloid-Z.125, 51–57 und 108–122 (1952).
Vettermann, W., and H. H.Paradies, 1977: Isolation and characterization of phosphofructokinase fromDunaliella salina. Arch. Biochem. Biophys., in press.
Woodward, C. K., L. M. Eillis, andA. Rosenberg, 1975 a: J. biol. Chem250, 432–439, 440–444. I. Solvent Acessibility in Folded Proteins, 432–439, J. biol. Chem.250. II. The Solvent Dependence of Hydrogen Exchange Kinetics of Folded Proteins 440–444.
Author information
Authors and Affiliations
Additional information
Fachrichtung Biochemie der Pflanzen und
Fachrichtung Feinstrukturforschung und Elektronenmikroskopie.
Rights and permissions
About this article
Cite this article
Paradies, H.H., Vettermann, W. & Werz, G. Shape of phosphofructokinase fromEscherichia coli in solution. Protoplasma 92, 43–56 (1977). https://doi.org/10.1007/BF01280199
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01280199