Summary
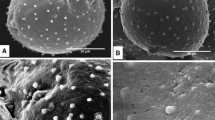
In order to compare cell wall formation in gymnosperm pollen with that in angiosperm pollen, the distribution of cell wall constituents in the pollen grain and pollen tube ofPinus densiflora was studied immunocytochemically with monoclonal antibodies JIM 5 (against non- or poorly esterified pectin), JIM 7 (against highly esterified pectin), JIM 13 (against arabinogalactan proteins, AGPs), and LM 2 (against AGPs containing glucuronic acid). In the pollen grain wall, only the outer layer of the intine was labeled with JIM 5 and weakly with JIM 7. The tube wall was scarcely labeled with JIM 5 and very weakly labeled with JIM 7. In contrast, the whole of both the intine and the tube wall was strongly labeled with JIM 13 and LM 2, and the generative-cell wall was also labeled only with LM 2. The hemicellulose B fraction, which is the main polysaccharide fraction from the pollen tube wall, reacted strongly with JIM 13 and especially LM 2, but not with antipectin antibodies. These results demonstrate that the wall constituents and their localization inP. densiflora pollen are considerably different from those reported in angiosperm pollen and suggest that the main components of the cell wall ofP. densiflora pollen are arabinogalactan and AGPs containing glucuronic acid.
Similar content being viewed by others
Abbreviations
- AGPs:
-
arabinogalactan proteins
- ELISA:
-
enzymelinked immunosorbent assay
- MAbs:
-
monoclonal antibodies
References
Boubeng HO (1965) Polysaccharides in pollen. Acta Chem Scand 19: 953–963
Cox GC, Juniper BE (1973) Autoradiographic evidence for para-mural-body function. Nature New Biol 243: 116–117
Cresti M, Pacini E, Canpolini F, Sarfatti G (1977) Germination and early tube development in vitro ofLycopersicum perufianum pollen: ultrastructural features. Planta 136: 239–247
Geitmann A, Hudák J, Vennigerholz F, Walles B (1995a) Immunogold localization of pectin and callose in pollen grains and pollen tubes ofBrugmansia suaveolens: implications for the self-incompatibility reaction. J Plant Physiol 147: 225–235
—, Li YQ, Cresti M (1995b) Ultrastructural immuno-localization of periodic pectin depositions in the cell wall ofNicotiana tabacum pollen tubes. Protoplasma 187: 168–171
Hara A, Yamashita H, Kobayashi A (1977) Isolation of a polysaccharide from the inner cell wall, intine, of pollen ofCryptomeria japonica. Plant Cell Physiol 18: 381–386
Hasegawa Y, Nakamura S, Nakamura N (1996) Immunocyto-chemical localization of callose in the germinated pollen ofCamellia japonica. Protoplasma 194: 133–139
Heslop-Harrison J. Heslop-Harrison Y (1980) The pollen-stigma interaction in the grasses I: fine-structure and cytochemistry of the stigmas ofHordeum andSecale. Acta Bot Neerl 29: 261–276
Hiratsuka R, Terasaka O (1996) Growth of pollen tube in gymnosperms I: the vesicles inPinus pollen tube. Jpn J Palynol 42: 93–99
Jaugh GY, Lord EM (1996) Localization of pectins and arabino-galactan-proteins in lily (Lilium longiflorum L.) pollen tubes and style, and their possible roles in pollination. Planta 199: 251–261
Knox JP (1967) The use of antibodies to study the architecture and developmental regulation of plant cell walls. Int Rev Cytol 171: 79–120
—, Linstead PJ, King J, Cooper C, Roberts K (1990) Pectin esterification is spatially regulated both within cell walls and between developing tissues of root apices. Planta 181: 512–521
— —, Peart J, Cooper C, Roberts K (1991) Developmentally regulated epitopes of cell surface arabinogalactan proteins and their relation to root tissue pattern formation. Plant J 1: 317–326
Li YQ, Bruun L, Pierson E, Cresti M (1992) Periodic deposition of arabinogalactan epitopes in the cell wall of pollen tubes ofNicotiana tabacum L. Planta 188: 532–538
—, Chen F, Linskens HF, Cresti M (1994) Distribution of unesterified and esterified pectins in the cell walls of pollen tubes of flowering plants. Sex Plant Reprod 7: 145–152
—, Faleri C, Geitman A, Zhang HQ, Cresti M (1995) Immunogold localization of arabinogalactan proteins, unesterified and esterified pectins in pollen grains and pollen tubes ofNicotiana tabacum L. Protoplasma 189: 26–36
—, Moscatelli A, Cai G, Cresti M (1997) Functional interactions among cytoskeleton, membranes, and cell wall in the pollen tubes of flowering plants. Int Rev Cytol 176: 133–199
Marchant R, Robards AW (1968) Membrane systems associated with the plasmalemma of plant cells. Ann Bot 32: 457–471
Mogami N, Nakamura S, Nakamura N (1997) Properties of polysac-charides from the pollen cell wall ofPinus densiflora. Jpn J Palynol 43: 1–8
Nakamura N, Yoshida K (1980a) A pectic substance extracted from the pollen tube wall ofCamellia japonica. Jpn J Palynol 43: 1–8
—, Yoshida K, Suzuki H (1980b) Hemicellulose of the pollen tube wall ofCamellia japonica, Plant Cell Physiol 21: 1383–1390
Smallwood M, Yates EA, Willates WGT, Martin H, Knox JP (1996) Immunochemical comparison of membrane-associated and secreted arabinogalactan-protein in rice and carrot. Planta 198: 452–459
Stanley RG, Linskens HF (1974) Pollen. Springer, Berlin Heidelberg New York Tokyo
Terasaka O, Niitsu T (1994a) Differential roles of microtubule and actin-myosin cytoskeleton in the growth ofPinus pollen tubes. Sex Plant Reprod 7: 264–272
— — (1994b) Kinesin localized in the pollen tube tips ofPinus densiflora. Jpn J Palynol 40: 1–6
Van Aelst AC, Van Went JL (1992) Ultrastructural immuno-localization of pectins and glycoproteins inArabidopsis thaliana pollen grains. Protoplasma 168: 14–19
Vandenbosch KA, Bradley DJ, Knox JP, Perotto S, Buchter GW, Brewin NJ (1989) Common components of the infection thread matrix and the intercellular space identified by immuno-cyto-chemical analysis of pea nodules and uninfected roots. EMBO J 8: 335–342
Win AHN de, Knuiman B, Pierson ES, Geurts H, Kengen HMP, Derksen J (1996) Development and cellular organization ofPinus sylvestris pollen tubes. Sex Plant Reprod 9: 93–101
Zhang GF, Staehelin LA (1992) Functional compartmentation of the Golgi apparatus of plant cells. Plant Physiol 99: 1070–1083
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mogami, N., Nakamura, S. & Nakamura, N. Immunolocalization of the cell wall components inPinus densiflora pollen. Protoplasma 206, 1–10 (1999). https://doi.org/10.1007/BF01279247
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01279247