Summary
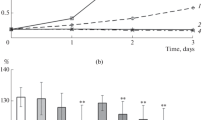
The mechanism of salt tolerance was studied using isolated internodal cells of the charophyteNitellopsis obtusa grown in fresh water. When 100 mM NaCl was added to artificial pond water (0.1 mM each of NaCl, KC1, CaCl2), no cell survived for more than one day. Within the first 30 minutes, membrane potential (Em) depolarized and membrane resistance (Rm) decreased markedly. Simultaneously, cytoplasmic Na+ increased and K+ decreased greatly. At steady state the increase in Na+ content was roughly equal to the decrease in K+ content. The Cl− content of the cytoplasm did not change. These results suggest that Na+ enters the cytoplasm by exchange with cytoplasmic K+. Both the entry of Na+ and the exit of K+ are assumed to be passive and the latter being caused by membrane depolarization. Vacuolar K+, Na+, and Cl− remained virtually constant, suggesting that rapid influx of Na+ from the cytoplasm did not occur.
In 100 mM NaCl containing 10 mM CaCl2, membrane depolarization, membrane resistance decrease and changes in cytoplasmic [Na+] and [K+] did not occur, and cells survived for many days. When cells treated with 100 mM NaCl were transferred within 1 hour to 100 mM NaCl containing 10 mM CaCl2, Em decreased, Rm increased, cytoplasmic Na+ and K+ returned to their initial levels, and cells survived. Two possible mechanisms for the role of Ca2+ in salt tolerance inNitellopsis are discussed; one a reduction in plasmalemma permeability to Na+ and the other a stimulation of active Na+-extrusion.
Similar content being viewed by others
References
Abe S, Takeda J (1986) The membrane potential of enzymatically isolatedNitella expansa protoplasts as compared with their intact cells. J Exp Bot 37: 238–252
Ben-Hayyim G, Kochba J (1983) Aspects of salt tolerance in a NaCl-selected stable cell line ofCitrus sinensis. Plant Physiol 72: 685–690
Ben-Hayyim G, Spiegel-Roy P, Neumann H (1985) Relation between ion accumulation of salt-sensitive and isolated stable salt-tolerant cell lines ofCitrus aurantium. Plant Physiol 78: 144–148
Binzel ML, Hasegawa PM, Handa AK, Bressan RA (1985) Adaptation of tobacco cells to NaCl. Plant Physiol 79: 118–125
Cramer GR, Läuchli A, Polito VS (1985) Displacement of Ca+ by Na+ from the plasmalemma of root cells. A primary response to salt stress? Plant Physiol 79: 207–211
Evans HJ, Sorger GJ (1966) Role of mineral elements with emphasis on the univalent cations. Ann Rev Plant Physiol 17: 47–76
Epstein E (1961) The essential role of calcium in selective cation transport by plant cells. Plant Physiol 36: 437–444
Flowers TJ, Troke PF, Yeo AR (1977) The mechanism of salt tolerance in halophytes. Ann Rev Plant Physiol 28: 89–121
Greenway H, Munns R (1980) Mechanisms of salt tolerance in nonhalophytes. Ann Rev Plant Physiol 31: 149–190
Hope AB, Walker NA (1975) The physiology of giant algal cells. Cambridge University Press, Cambridge.
Kent LM, Läuchli A (1985) Germination and seedling growth of cotton: salinity—calcium interaction. Plant Cell Env 8: 155–159
Kishimoto U (1959) Electrical characteristics ofChara corallina. Ann Rep Sci Works Fac Sci Osaka Univer 7: 115–146
Kishimoto U, Akabori H (1959) Protoplasmic streaming of an internodal cell ofNitella flexilis. J Gen Physiol 42: 1167–1183
Kishimoto U, Tazawa M (1965) Ionic composition of the cytoplasm ofNitella flexilis. Plant Cell Physiol 6: 507–518
Lahaye PA, Epstein E (1969) Salt toleration by plants: enhancement with calcium. Science 166: 395–396
Lahaye PA, Epstein E (1971) Calcium and salt toleration by bean plants. Physiol Plant 25: 213–218
Lener HR (1985) Adaptation to salinity at the plant cell level. Plant and Soil 89: 3–14
Mailman DS, Mullins LJ (1966) The electrical measurement of chloride fluxes. Aust J Biol Sci 19: 385–398
Mimura T, Shimmen T, Tazawa M (1984) Adenine-nucleoide levels and metabolism-dependent membrane potential in cells ofNitellopsis obtusa Groves. Planta 162: 77–84
Munns R, Greenway H, Kirst GO (1983) Halotolerant eukaryotes. In: Encycl Pl Physiol New Series, 12C. Springer, Berlin Heidelberg New York, pp 59–135
Ponnamperuma FH (1984) Role of cultivar tolerance in increasing rice production on saline lands. In:Staples RC, Toenniessen GH (eds) Salinity tolerance in plants. Wiley Interscience, New York, pp 255–271
Sakano K, Tazawa M (1984) Intracellular distribution of free amino acids between the vacuolar and extravacuolar compartments in internodal cells ofChara australis. Plant Cell Physiol 25: 1477–1486
Shannon MC (1984) Breeding, selecting, and the genetics of salt tolerance. In:Staples RC, Toenniessen GH (eds) Salinity tolerance in plants. Wiley Interscience, New York, pp 231–254
Spanswick RM, Stolark J, Williams EJ (1967) The membrane potential ofNitella translucens. J Exp Bot 18: 1–16
Srivastava JP, Juna S (1984) Screening wheat and barley germplasm for salt tolerance. In:Staples PC, Toenniessen GH (eds) Salinity tolerance in plants. Wiley Interscience, New York, pp 273–284
Takeshige K, Shimmen T, Tazawa M (1986) Quantitative analysis of ATP-dependent H+ efflux and pump current driven by an electrogenic pump inNitellopsis obtusa. Plant Cell Physiol 27: 337–348
Tazawa M (1972) Membrane characteristics as revealed by water and ionic relations of algal cells. Protoplasma 75: 427–460
Tazawa M, Kishimoto U, Kikuyama M (1974) Potassium, sodium, and chloride in the protoplasm of characeae. Plant Cell Physiol 15: 103–110
Umrath K (1933) Der Erregungsvorgang beiNitella mucronata. Protoplasma 17: 258–300
Viets FG (1944) Calcium and other polyvalent cations as accelerators of ion accumulation by excised barley roots. Plant Physiol 19: 466–480
Watad AA, Reinhold L, Lerner HR(1983) Comparison between a stable NaCl-selectedNicotiana cell line and the wild type. K+, Na+, and proline pool as a function of salinity. Plant Physiol 73: 624–629
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Katsuhara, M., Tazawa, M. Salt tolerance inNitellopsis obtusa . Protoplasma 135, 155–161 (1986). https://doi.org/10.1007/BF01277008
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01277008