Summary
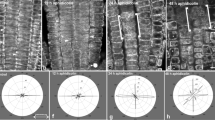
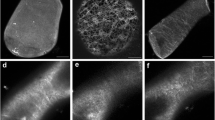
To examine whether preprophase microtubule band (PPB) organization occurs by rearrangement of pre-existing, or by assembly of new microtubules (Mts), we treated root cells ofTriticum turgidum with taxol, which stabilizes pre-existing Mts by slowing their depolymerization. With taxol early preprophase cells failed to form a normal PPB and PPB narrowing was prevented in cells that had already formed a wide one. The PPB became persistent in prometaphase cells and the formation of multipolar prophase-prometaphase spindles was induced. These data favour the suggestion that PPB formation and narrowing, as well as prophase spindle development, are dynamic processes depending on continuous Mt assembly at the PPB site and in the perinuclear cytoplasm.
Similar content being viewed by others
Abbreviations
- Mt:
-
microtubule
- MTOC:
-
microtubule organizing centre
- PPB:
-
preprophase microtubule band
- DMSO:
-
dimethyl sulfoxide
References
Apostolakos P, Galatis B (1985) Studies on the development of the air pores and air chambers ofMarchantia paleacea. III. Microtubule organization in preprophase-prophase initial aperture cells-formation of incomplete preprophase microtubule bands. Protoplasma 128: 120–135
— — (1987) Induction, polarity and spatial control of cytokinesis in some abnormal subsidiary cell mother cells ofZea mays. Protoplasma 140: 26–42
— — (1992) Patterns of microtubule organization in two polyhedral cell types in the gametophyte of the liverwortMarchantia paleacea Bert. New Phytol 122: 165–178
Asada T, Sonobe S, Shibaoka H (1991) Microtubule translocation in the cytokinetic apparatus of cultured tobacco cells. Nature 350: 238–241
Bajer AS, Cypher C, Molé-Bajer J, Howard HM (1982) Taxol in mitosis I. Pushing force by assembly of microtubules. Proc Natl Acad Sci USA 79: 6569–6573
Baskin TI, Cande WZ (1990) The structure and function of the mitotic spindle in flowering plants. Annu Rev Plant Physiol Plant Mol Biol 41: 277–315
Cho S-O, Wick SM (1989) Microtubule orientation during stomatal differentiation in grasses. J Cell Sci 92: 581–594
Cleary AL, Hardham AR (1988) Depolymerization of microtubule arrays in root tips by oryzalin and their recovery with modified nucleation patterns. Can J Bot 66: 2353–2366
— — (1989) Microtubule organization during development of stomatal complexes ofLolium rigidum. Protoplasma 149: 67–81
—, Gunning BES, Wasteneys GO, Hepler PK (1992) Microtubule and F-actin dynamics at the division site in livingTradescantia stamen hair cells. J Cell Sci 103: 977–988
Eleftheriou EP, Palevitz BA (1992) The effect of cytochalasin D on preprophase band organization in root tip cells ofAllium, J Cell Sci 103: 989–998
Falconer MM, Seagull RW (1985) Xylogenesis in tissue culture: taxol effect on microtubule reorientation and lateral association in differentiating cells. Protoplasma 128: 157–166
Falconer MM, Donaldson G, Seagull RW (1988) MTOCs in higher plant cells: an immunofluorescent study of microtubule assembly sites following depolymerization by APM. Protoplasma 144: 46–55
Flanders DJ, Rawlins DJ, Shaw PJ, Lloyd CW (1990) Nucleus-associated microtubules help determine the division plane of plant epidermal cells: avoidance of four-way junctions and the role of cell geometry. J Cell Biol 110: 1111–1122
Fukuda H, Kobayashi H (1989) Dynamic organization of the cytoskeleton during tracheary-element differentiation. Dev Growth Differ 31: 9–16
Galatis B (1982) The organization of microtubules in guard cell mother cells ofZea mays. Can J Bot 60: 1148–1166
—, Apostolakos P (1991) Patterns of microtubule reappearance in root tip cells ofVigna sinensis recovering from a colchicine treatment. Protoplasma 160: 131–143
— —, Katsaros B (1983) Synchronous organization of two preprophase microtubule bands in subsidiary cell mother cells of someTriticum species. Protoplasma 117: 24–39
Gunning BES (1992) Use of confocal microscopy to examine transitions between successive microtubule arrays in the plant cell division cycle. In: Shibaoka H (ed) Proceedings of the International Symposium on the Cellular Basis of Growth and Development in Plants. Toyonaka, Osaka, November 26–28, 1991, pp 45–55
—, Sammut M (1990) Rearrangement of microtubules involved in establishing cell division planes start immediately after DNA synthesis and are completed just before mitosis. Plant Cell 2: 1273–1282
—, Hardham AR, Hughes JE (1978) Evidence for initiation of microtubules in discrete regions of the cell cortex inAzolla root-tip cells, and an hypothesis on the development of cortical arrays of microtubules. Planta 143: 161–179
Hasezawa S, Marc J, Palevitz BA (1991) Microtubule reorganization during the cell cycle in synchronized BY-2 tobacco suspensions. Cell Motil Cytoskeleton 18: 94–106
Hepler PK, Cleary AL, Gunning BES, Wadsworth P, Wasteneys GO, Zhang DH (1993) Cytoskeletal dynamics in living plant cells. Cell Biol Int 17: 127–142
Kuss-Wymer CL, Cyr RJ (1992) Tobacco protoplasts differentiate into elongate cells without net microtubule depolymerization. Protoplasma 168: 64–72
Liu B, Marc J, Joshi HC, Palevitz BA (1993) A γ-tubulin-related protein associated with the microtubule arrays of higher plants in a cell cycle-dependent manner. J Cell Sci 104: 1217–1228
Mazia D (1984) Centrosomes and mitotic poles. Exp Cell Res 153: 1–15
— (1987) The chromosome cycle and the centrosome cycle in the mitotic cycle. Int Rev Cytol 100: 49–92
Melan MA (1990) Taxol maintains organized microtubule patterns in protoplasts which lead to the resynthesis of organized cell wall microfibrils. Protoplasma 153: 169–177
Mineyuki Y, Palevitz BA (1990) Relationship between preprophase band organization, F-actin and the division site inAllium. Fluorescence and morphometric studies on cytochalasin-treated cells. J Cell Sci 97: 283–295
—, Marc J, Palevitz BA (1989) Development of the preprophase band from random cytoplasmic microtubules in guard cell mother cells ofAllium cepa L. Planta 178: 291–296
Molé-Bajer J, Bajer AS (1983) Action of taxol on mitosis: modification of microtubule arrangements and function of the mitotic spindle inHaemanthus endosperm. J Cell Biol 96: 527–540
Morejohn LC, Fosket DE (1991) The biochemistry of compounds with anti-microtubule activity in plant cells. Pharmacol Ther 51: 217–230
Mullinax JB, Palevitz BA (1989) Microtubule reorganization accompanying preprophase band formation in guard mother cells ofAvena sativa L. Protoplasma 149: 89–94
Oakley BR (1992) γ-Tubulin: the microtubule organizer? Trends Cell Biol 2: 1–5
Palevitz BA (1991) Potential significance of microtubule rearrangement, translocation and reutilization in plant cells. In: CW Lloyd (ed) The cytoskeletal basis of plant growth and form. Academic Press, London, pp 45–55
Panteris E, Galatis B, Apostolakos P (1991) Patterns of cortical and perinuclear microtubule organization in meristematic root cells ofAdiantum capillus veneris. Protoplasma 165: 173–188
Seagull RW (1990) The effects of microtubule and microfilament disrupting agents on cytoskeletal arrays and wall deposition in developing cotton fibers. Protoplasma 159: 44–59
—, Falconer MM (1991) In vitro xylogenesis. In: CW Lloyd (ed) The cytoskeletal basis of plant growth and form. Academic Press, London, pp 183–194
Staiger CJ, Lloyd CW (1991) The plant cytoskeleton. Curr Opin Cell Biol 3: 33–42
Wang H, Cutler AJ, Fowke LC (1989) High frequencies of preprophase bands in soybean protoplast cultures. J Cell Sci 92: 575–580
Weerdenburg C, Falconer MM, Setterfield G, Seagull RW (1986) Effects of taxol on microtubule arrays in cultured higher plant cells. Cell Motil Cytoskeleton 6: 469–478
Wick SM (1991) The preprophase band. In: Lloyd CW (ed) The cytoskeletal basis of plant growth and form. Academic Press, London, pp 231–244
—, Duniec J (1983) Immunofluorescence microscopy of tubulin and microtubule arrays in plant cells. I. Preprophase band development and concomitant appearance of nuclear envelope-associated tubulin. J Cell Biol 97: 235–243
— — (1984) Immunofluorescence microscopy of tubulin and microtubule arrays in plant cells. II. Transition between the preprophase band and the mitotic spindle. Protoplasma 122: 45–55
Wilson L, Jordan MA (1994) Pharmacological probes of microtubule function. In: Hyams JS, Lloyd CW (eds) Microtubules. Wiley-Liss, New York, pp 59–83
Yasuhara H, Sonobe S, Shibaoka H (1993) Effects of taxol on the development of the cell plate and of the phragmoplast in tobacco BY-2 cells. Plant Cell Physiol 34: 21–29
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Panteris, E., Apostolakos, P. & Galatis, B. The effect of taxol onTriticum preprophase root cells: preprophase microtubule band organization seems to depend on new microtubule assembly. Protoplasma 186, 72–78 (1995). https://doi.org/10.1007/BF01276938
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01276938