Summary
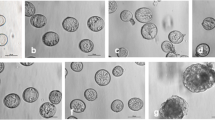
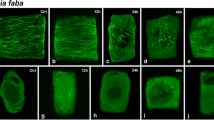
Effects of cycloheximide (CHM) on preprophase bands (PPBs) of microtubules (MTs) and on prophase spindle MTs in root tip cells of onion (Allium cepa L.) were examined. When root tip cells were treated with 36 μM CHM for 0.5–4 h, the population of cells with a PPB did not decrease markedly although the population of mitotic cells and that of prophase cells with a PPB gradually decreased to half of the control root tips. In prophase cells treated with 11 and 36 μM CHM for 2 h, the width of the PPB was 1.4 times broader than that in the prophase PPB without CHM. Electron microscopic observation on the cross section of the PPB showed that the number of MTs and the distance between adjacent MTs in prophase PPBs treated with CHM were similar to those in the early developmental stage of PPBs without CHM. The bipolar spindle, that appeared in late prophase was not seen in prophase cells treated with 11 μM or higher concentrations of CHM for 2 h. In order to examine differences of perinuclear MT arrangement between CHM treated and non-treated prophase cells, arrangement of perinuclear MTs was examined by confocal laser scanning microscopy. In control cells without CHM, MTs appeared on the nuclear surface with several “branched” or “cross over” type MT foci in the cytoplasm when broad PPB formation started. These MT foci were replaced by the “aster” type MT foci, from which several MTs radiated along the nuclear surface. The “aster” type MT foci gradually gathered to form a bipolar spindle. MTs connecting the spindle pole region and the PPB were seen in late prophase. In CHM-treated cells (11-360 μM for 2 h), “branched” and “cross over” type MT foci were prominent, even in prophase cells with well condensed chromosomes. Neither linkages of MTs between the spindle pole region and the PPB nor “aster” type MT foci were seen. These observations showed that CHM prevents the bundling of MTs in the PPB and also inhibits the formation of “aster” type MT foci that is essential for bipolar spindle development.
Similar content being viewed by others

References
Benbadis M-C, Levy F, Deysson MG (1974) Interruption de la mitose en prophase avec persistance de la membrane nucléaire sous I'influence du cycloheximide: etude ultrastructurale. C R Acad Sci Ser D 278: 1353–1355
Cho S-O, Wick SM (1989) Microtubule orientation during stomatal differentiation in grasses. J Cell Sci 92: 581–594
Cleary A, Hardham AR (1989) Microtubule organization during development of stomatal complexes inLolium rigidum. Protoplasma 149: 67–81
Colasanti J, Cho S-O, Wick S, Sundaresan V (1993) Localization of the functional p34cdc2 homolog of maize root tip and stomatal complex cells: association with predicted division sites. Plant Cell 5: 1101–1111
Cyr RJ (1994) Microtubules in plant morphogenesis: role of the cortical array. Annu Rev Cell Biol 10: 153–180
—, Palevitz BA (1989) Microtubule-binding proteins from carrot. I. Initial characterization and microtubules bundling. Planta 177: 245–260
Deckert J, Taranenko N, Gresshoff PM (1994) Cell cycle genes and their plant homologues. In: Gresshoff PM (ed) Plant genome analysis. CRC Press, Boca Raton, pp 169–193
Eleftheriou EP, Palevitz BA (1992) The effect of cytochalasin D on preprophase band organization in root tip cells ofAllium. J Cell Sci 103: 989–998
García-Herdugo G, Fernández-Gometz ME, Hidalgo J, Lopez-Saez JF (1974) Effects of protein synthesis inhibition during plant mitosis. Exp Cell Res 89: 336–342
Gunning BES (1992) Use of confocal microscopy to examine transitions between successive microtubule arrays in the plant cell division cycle. In: Shibaoka H (ed) Proceedings of the VII International Symposium in conjunction with the awarding of the International Prize for Biology. Cellular Basis of Growth and Development in Plants. Osaka University, Toyonaka, pp 145–155
—, Sammut M (1990) Rearrangements of microtubules involved in establishing cell division planes start immediately after DNA synthesis and are completed just before mitosis. Plant Cell 2: 1273–1282
Jiang C-J, Sonobe S (1993) Identification and preliminary characterization of a 65 kDa higher-plant microtubule-associated protein. J Cell Sci 105: 891–901
Lambert A-M (1980) The role of chromosomes in anaphase trigger and nuclear envelope activity in spindle formation. Chromosoma 76: 295–308
Mineyuki Y, Palevitz BA (1990) Relationship between preprophase band organization, F-actin and the division site inAllium. Fluorescence and morphometric studies on cytochalasin-treated cells. J Cell Sci 97: 283–295
—, Marc J, Palevitz BA (1988a) Formation of the oblique spindle in dividing guard mother cells ofAllium. Protoplasma 147: 200–203
—, Wick SM, Gunning BES (1988b) Preprophase bands of microtubules and cell cycle: kinetics and experimental uncoupling of their formation from the nuclear cycle in onion root-tip cells. Planta 174: 518–526
—, Marc J, Palevitz BA (1989) Development of the preprophase band from random cytoplasmic microtubules in guard mother cells ofAllium cepa L. Planta 178: 291–296
— — — (1991a) Relationship between the preprophase band, nucleus and spindle in dividingAllium cotyledon cells. J Plant Physiol 138: 640–649
—, Yamashita M, Nagahama Y (1991b) p34cdc2 kinase homologue in the preprophase band. Protoplasma 162: 182–186
—, Lida H, Anraku Y (1994) Loss of microtubules in the interphase cells of onion (Allium cepa L.) root tips from the cell cortex and their appearance in the cytoplasm after treatment with cycloheximide. Plant Physiol 104: 281–284
Mizuno K (1993) Microtubule-nucleation sites on nuclei of higher plant cells. Protoplasma 173: 77–85
Murray A, Hunt T (1993) The cell cycle: an introduction. WH Freeman, New York
Olszewska MJ, Marciniak K, Kuran H (1990) The timing of synthesis of proteins required for mitotic spindle and phragmoplast in partially synchronized root meristems ofVicia faba L. Eur J Cell Biol 53: 89–92
Ookata K, Hisanaga S, Okumura E, Kishimoto T (1933) Association of p34cdc2/cyclin B complex with microtubules in starfish oocytes. J Cell Sci 105: 873–881
Panteris E, Galatis B, Apostolakos P (1991) Patterns of cortical and perinuclear microtubule organization in meristematic root cells ofAdiantum capillus-veneris. Protoplasma 165: 173–188
Rose RJ (1970) Effect of cycloheximide on cell division in partially synchronized plant cells. Aust J Biol Sci 23: 573–583
Reynolds ES (1963) The use of lead citrate at high pH as an electronopaque stain in electron microscopy. J Cell Biol 17: 208–212
Schmit A-C, Vantrard M, De May J, Lambert A-M (1983) Aster-like microtubule centers establish spindle polarity during interphasemitosis transition in higher plant cells. Plant Cell Rep 2: 285–288
— —, Lambert A-M (1985) Microtubules and F-actin rearrangement during the initiation of mitosis in acentriolar higher plant cells. In: Ishikawa H, Hatano S, Sato H (eds) Cell motility: mechanism and regulation. University of Tokyo Press, Tokyo, pp 415–433
Smirnova EA, Bajer AS (1994) Microtubule converging centers and reorganization of the interphase cytoskeleton and the mitotic spindle in higher plantHaemanthus. Cell Motil Cytoskeleton 27: 219–233
Spurr AR (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26: 31–43
Stoppin V, Vantard M, Schmit A-C, Lambert A-M (1994) Isolated plant nuclei nucleate microtubule assembly: the nuclear surface in higher plants has centrosome-like activity. Plant Cell 6: 1099–1106
Webster PL (1973) Effect of cycloheximide on mitosis inVicia faba root-meristem cells. J Exp Bot 24: 239–244
Wick SM, Duniec J (1983) Immunofluorescence microscopy of tubulin and microtubule arrays in plant cells. I. Pre-prophase band development and concomitant appearance of nuclear envelope-associated tubulin. J Cell Biol 97: 235–243
— — (1984) Immunofluorescence microscopy of tubulin and microtubule arrays in plant cells. II. Transition between the preprophase band and the mitotic spindle. Protoplasma 122: 45–55
Seagull RW, Osborn M, Weber K (1981) Immunofluorescence microscopy of organized microtubule arrays in structurally stabilized meristematic plant cells. J Cell Biol 89: 685–690
Wilson GB (1950) Cytological effects of some antibiotics. J Hered 41: 227–231
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nogami, A., Suzaki, T., Shigenaka, Y. et al. Effects of cycloheximide on preprophase bands and prophase spindles in onion (Allium cepa L.) root tip cells. Protoplasma 192, 109–121 (1996). https://doi.org/10.1007/BF01273249
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01273249