Summary
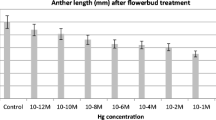
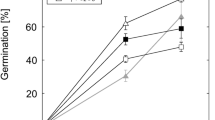
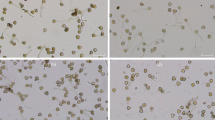
The influence of different concentrations of the heavy metals cadmium (Cd2+), cobalt (Co2+), copper (Cu2+), iron (Fe2+ and Fe3+), mercury (Hg2+), manganese (Mn2+), and zinc (Zn2+), plus aluminium (Al3+) (a toxic metal in polluted areas), on pollen germination and tube growth ofLilium longiflorum was investigated using light microscopy. Effects could be observed with 3 μM and 100 μM of heavy metal, added as chloride salts to the medium. Cd2+, Cu2+, and Hg2+, showed the greatest toxicity, whereas germination and growth rate was less affected by Mn2+. Affected tubes showed swelling of the tip region. Tubes treated with Cd2+, Co2+, Fe2+, Fe3+, Hg2+, and Mn2+ were also prepared for ultrastructural studies. In all cases, the main effect was abnormal cell wall organization, mostly at the tip, where round, fibrillar aggregates, the shape and size of secretory Golgi vesicles were formed. They built up a loose network which could be up to 10 μm thick compared to untreated tubes where the cell wall was composed of thin layers of long fibrils and about 100 nm thick. Cd2+ was the only metal which produced effects at the intracellular level: organelle distribution within the tip region appeared disorganized. A general mechanism of heavy metal action on pollen tube growth is discussed.
Similar content being viewed by others
References
Chukumura (1993) Comparison of the accumulation of cadmium, lead and zinc in cultivated and wild plant species in the derelict Enyigbe lead-zinc mine. Toxicol Environ Chem 38: 167–173
Clarkson DT (1967) Interactions between aluminium and phosphorus on root surfaces and cell wall synthesis. Plant Soil 27: 347–356
Faulstich H, Stournaras C (1985) Potentially toxic concentrations of triethyl lead in Black Forest rainwater samples. Nature 317: 714–715
Foissner I (1990) Wall appositions induced by ionophore A 23187, CaCl2 LaCl3, and nifedipine in characean cells. Protoplasma 154: 80–90
Franke WW, Herth W, van der Woude WJ, Morré DJ (1972) Tubular and filamentous structures in pollen tubes: possible involvement as guide elements in protoplasmic streaming and vectorial migration of secretory vesicles. Planta 105: 317–341
Herth W, Franke WW, Bittiger H, Kuppel A, Keilich G (1974) Alkali-resistant fibrils of β-1,3- and β-1,4-glucans: structural polysaccharides in the pollen tube wall ofLilium longiflorum. Cytobiol 9: 344–367
Heslop-Harrison J (1987) Pollen germination and pollen-tube growth. Int Rev Cytol 107: 1–78
Heumann H-G (1987) Effects of heavy metal on growth and ultrastructure ofChara vulgaris. Protoplasma 136: 37–48
Hinsley TD (1989) Uptake of cadmium by crop plants grown on sludge-amended soil. In: Cadmium 79-edited proceedings, Second International Cadmium Conference, Cannes, France, pp 83–90
Jarvis MC (1982) The proportion of calcium-bound pectin in plant cell walls. Planta 154: 344–346
Joos U, van Aken J, Kristen U (1994) Microtubules are involved in maintaining the cellular polarity in pollen tubes ofNicotiana sylvestris. Protoplasma 179: 5–15
Kauss H (1987) Some aspects of calcium-dependent regulation in plant metabolism. Annu Rev Plant Physiol 38: 47–72
Kristen U, Hoppe U, Pape W (1993) The pollen tube growth test: a new alternative to the Draize eye irritation assay. J Soc Cosmet Chem 44: 153–162
Lane SD, Martin ES, Garrod JF (1978) Lead toxicity effects on indole-3-ylacetic acid-induced cell elongation. Planta 144: 79–84
Malone C, Koeppe DE, Miller RJ (1974) Localization of lead accumulated by corn plants. Plant Physiol 53: 388–394
Matsumoto H, Morimura S, Takahashi E (1977) Less involvement of pectin in the precipitation of aluminium in pea roots. Plant Cell Physiol 18: 325–335
McKinney J (1993) Metals bioavailability and disposition kinetics research needs workshop July 18–19, 1990. Toxicol Environ Chem 38: 1–71
Pellegrini LP, Pellegrini M, Delivopoulos S, Berail G (1991) The effects of cadmium on the fine structure of the brown algaCystoseira barbata forma repens Zinova et Kalugina. Br Phycol J26: 1–8
Read SM, Clarke AE, Bacic A (1993) Stimulation of growth of culturedNicotiana tabacum W 38 pollen tubes by poly(ethylene glycol) and Cu(II) salts. Protoplasma 177: 1–14
Reiss H-D, Herth W (1979) Calcium ionophore A-23187 affects localized wall secretion in the tip region of pollen tubes ofLilium longiflorum. Planta 145: 225–232
— —, Schnepf E, Nobiling R (1983) The tip-to-base calcium gradient in pollen tubes ofLilium longiflorum measured by proton-induced X-ray emission (PIXE). Protoplasma 115: 153–159
Röderer G (1984) Toxic effects of plant organisms. In: Grandjean P (ed) Biological effects of organolead compounds. CRC Press, Boca Raton, pp 63–95
—, Reiss H-D (1988) Different effects of inorganic and triethyl lead on growth and ultrastructure of lily polen tubes. Protoplasma 144: 101–109
Schlüpmann H, Bacic A, Read SM (1993) A novel callose synthase from pollen tubes ofNicotiana. Planta 191: 470–481
Sharpe V, Denny P (1976) Electron microscope studies on the absorption and localization of lead in the leaf tissue ofPotomogeton pectinatus L. J Exp Bot 27: 1155–1162
Steer MW, Steer JM (1989) Pollen tube tip growth. New Phytol 111: 323–358
Tepfer M, Taylor IEP (1981) The interaction of divalent cations with pectic substances and their influence on acid-induced cell wall loosening. Can J Bot 59: 1522–1525
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sawidis, T., Reiss, H.D. Effects of heavy metals on pollen tube growth and ultrastructure. Protoplasma 185, 113–122 (1995). https://doi.org/10.1007/BF01272851
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01272851