Abstract
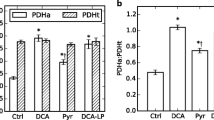
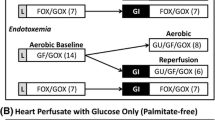
High levels of fatty acids decrease the extent of mechanical recovery of hearts reperfused following a transient period of severe ischemia. Glucose oxidation rates during reperfusion are low under these conditions, which can result in a decreased recovery of mechanical function. Stimulation of glucose oxidation with the carnitine palmitoyl transferase I inhibitor, Etomoxir, or by directly stimulating pyruvate dehydrogenase activity with dichloroacetate (DCA) results in an improvement in mechanical function during reperfusion of previously ischemic hearts. Addition of DCA (1 mM) to hearts perfused with 11 mM glucose and 1.2 mM palmitate results in an increase in contribution of glucose oxidation to overall ATP production from 6 to 23%, with a parallel decrease in that of fatty acid oxidation from 90 to 69%. In aerobic hearts, endogenous myocardial triglycerides are an important source of fatty acids for β-oxidation. Using hearts in which the myocardial triglycerides were pre-labeled, the contribution of both endogenous and exogenous fatty acid oxidation to myocardial ATP production was determined in hearts perfused with 11 mM glucose, 1.2 mM palmitate and 500 µU/ml insulin. In hearts reperfused following a 30 min period of global no flow ischemia, 91.9% of ATP production was derived from endogenous and exogenous fatty acid oxidation, compared to 87.7% in aerobic hearts. This demonstrates that fatty acid oxidation quickly recovers following a transient period of severe ischemia. Furthermore, therapy aimed at overcoming fatty acid inhibition of glucose oxidation during reperfusion of ischemic hearts appears to be beneficial to recovery of mechanical function.
Similar content being viewed by others
References
Opie LH: Metabolism of free fatty acids, glucose, and catecholamines in acute myocardial infarction. Am J Cardiol 36: 938–953, 1975
Vik-Mo H, Mjos OD: Influence of free fatty acids on myocardial oxygen consumption and ischemic injury. Am J Cardiol 48: 361–365, 1981
Liedtke AJ, Nellis SH, Neely JR: Effects of excess free fatty acids on mechanical and metabolic function in normal and ischemic myocardium in swine. Circ Res 43: 652–661, 1978
Mjos OD: Effect of free fatty acids on myocardial function and oxygen consumption in intact dogs. J Clin Invest 50: 1386–1389, 1978
Johnson DL, Lewandowski ED: Fatty acid metabolism and contractile function in the reperfused myocardium. Multinuclear NMR studies of isolated rabbit hearts. Cite Res 68: 714–725, 1991
Lopaschuk GD, Wall SR, Olley PM, Davies NJ: Etomoxir, a carnitine palmitoyltransferase I inhibitor, protects hearts from fatty acid-induced ischemic injury independent of changes in long chain acylcarnitine. Circ Res 63: 1036–1043, 1988
Lopaschuk GD, Spafford MA, Davies NJ, Wall SR: Glucose and palmitate oxidation in isolated working rat hearts reperfused after a period of transient global ischemia. Circ Res 66: 546–553, 1990
Oliver MF, Kurien VA, Greenwood TW: Relation between serum-free-fatty acids and arrythmia and death after myocardial infarction. Lancet 1: 71–715, 1968
Svensson S, Svedjeholm R, Ekroth R, Milocco I, Nilsson F, Sabel KG, William-Olsson G: Trauma metabolism and the heart. Uptake of substrates and effects of insulin early after cardiac operations. J Thorac Cardiovas Surg 99: 1063–1073, 1990
Owen P, Dennis S, Opie LH: Glucose flux rate regulates onset of ischemic contracture in globally underperfused rat hearts. Circ Res 66: 344–354, 1990
McVeigh JJ, Lopaschuk GD: Dichloroacetate stimulation of glucose oxidation improves recovery of ischemic rat hearts. Am J Physiol 259: H1070-H1085, 1990
Schwaiger M, Schelbert HR, Ellison D, Hansen H, Yeatman L, Vinten-Johansen J, Selin C, Barrio J, Phelps ME: Sustained regional abnormalities in cardiac metabolism after transient ischemia in the chronic dog model. J Am Coll Cardiol 6: 311–320, 1985
Teoh KH, Mickle DAG, Weisel RD, Fremes SE, Christakis GT, Romaschin AD, Harding RS, Madonik MM, Ivanov J: Decreased postoperative myocardial fatty acid oxidation. J Surg Res 44: 36–44, 1988
Myears DW, Sabel BE, Bergmann SR: Substrate use in ischemic and reperfused canine myocardium: Quantitative considerations. Am J Physiol 253: H107-H114, 1987
Liedtke AJ, Demaison L, Eggleston AM, Cohen LM, Nellis SH: Changes in substrate metabolism and effects of excess fatty acids in reperfused myocardium. Circ Res 62: 535–542, 1988
Saddik M, Lopaschuk GD: Myocardial triglyceride turnover and contribution to energy substrate utilization in isolated working rat hearts. J Biol Chem 266: 8162–8170, 1991
KerbeyAL, Randle PJ, Cooper RH, Whitehouse S, Pask HT, Denton RM: Regulation of pyruvate dehydrogenase in rat heart. Biochem J 154: 327–348, 1976
Newsholme EA, Randle PJ, Manchester KL: Inhibition of the phosphofructokinase reaction in perfused rat heart by respiration of ketone bodies, fatty acids and pyruvate. Nature 193: 270–271, 1962
Turnbull DM, Bartlett K, Younan SIM, Sherratt SA: The effects of 2(5(4-chlorophenyl)pentyl)oxirane-2-carbonyl-CoA on mitochondrial oxidations. Biochem Pharmacol 33: 475–481, 1984
Tutwiler GF, Mohrbacher R, Ho W: Methyl 2-tetradecyl-glycidate, an orally effective hypoglycaemic agent that inhibits long-chain fatty acid oxidation selectively. Diabetes 28: 511–519, 1979
McAllister A, Allison SP, Randle PJ: Effects of dichloroacetate on the metabolism of glucose, pyruvate, acetate, 3-hydroxybutyrate and palmitate in rat diaphragm and heart musclein vitro and on extraction of glucose, lactate, pyruvate and free fatty acids by dog heartsin vivo. Biochem J 134: 1067–1081, 1973
Stacpoole PW: The pharmacology of dichloroacetate. Metabolism 38: 1124–1144, 1989
Patel MS, Roche TE: Molecular biology and biochemistry of pyruvate dehydrogenase complexes. FASEB J 4: 3224–3233, 1990
Bunger R, Mallet RT, Hartman DA: Pyruvate-enhanced phosphorylation potential and inotropism in normoxic and postischemic isolated working heart. Eur J Biochem 180: 221–233, 1989
Mallet RT, Hartman DA, Bunger R: Glucose requirement for postischemic recovery of perfused working heart. Eur J Biochem 188: 481–493, 1990
Zimmer SD, Ugurbil K, Michurski SP, Mohanakrishnan P, Ulstad VK, Foker JE, From AHL: Alterations in oxidative function and respiratory regulation in the post-ischemic myocardium. J Biol Chem 264: 12402–12411, 1989
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lopaschuk, G.D., Saddik, M. The relative contribution of glucose and fatty acids to ATP production in hearts reperfused following ischemia. Mol Cell Biochem 116, 111–116 (1992). https://doi.org/10.1007/BF01270577
Issue Date:
DOI: https://doi.org/10.1007/BF01270577