Abstract
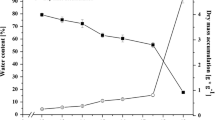
InRicinus communis L. (castor bean) endosperms, two classes of Late Embryogenesis Abundant (Lea) transcripts were first detected during mid-development (at 30–35 days after pollination, DAP) and peaked at 50 DAP, just prior to the onset of desiccation. Most of the Class 1 mRNAs declined substantially during desiccation itself; Class 11 mRNAs remained abundant in the mature dry (60 DAP) seed. Following imbibition, allLea mRNAs abundant in the mature dry seed declined rapidly (within 5–24 h). Premature drying of developing 35-DAP seeds resulted in the loss of storage-protein mRNAs (Leg B Mat I); following rehydration, mRNAs encoding post-germinative proteins (Germ D91, D30 and D38) increased in the endosperm. TheLea mRNAs present in the developing fresh seed at 35 DAP were preserved, but did not increase in response to premature desiccation; upon rehydration theseLea mRNAs declined within 5 h. During seed development, substantial changes occurred in the synthesis of a subset of LEA proteins referred to as ‘dehydrins’; in particular, new dehydrin polypeptides were induced between 40 and 60 DAP. Such proteins were not as evident in prematurely dried endosperms. In contrast to the rapid loss ofLea mRNAs during germination, many of the dehydrin proteins abundant in the dried seed persisted following imbibition or rehydration.
Similar content being viewed by others
Abbreviations
- DAP:
-
days after pollination
- DPA:
-
days postanthesis
- HAI:
-
hours after imbibition
- LEA:
-
late embryogenesis abundant
- Mr :
-
relative molecular mass
References
Bewley JD, Marcus A (1990) Gene expression in seed development and germination. In: Cohn WE, Moldave K (eds) Progress in nucleic acid research and molecular biology, vol 38. Academic Press, Orlando, Florida, pp 165–193
Bewley JD, Oliver MJ (1992) Desiccation tolerance in vegetative plant tissues and seeds: Protein synthesis in relation to desiccation and a potential role for protection and repair mechanisms. In: Somero GN, Osmond CB, Bolis CL (eds) Water and life: comparative analysis of water relationships at the organismic, cellular and molecular levels. Springer-Verlag, Berlin, Heidelberg, pp 141–160
Bradford M (1976) A rapid method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254
Bradford KJ, Chandler PM (1992) Expression of “dehydrin-like” proteins in embryos and seedlings ofZizania palustris andOryza sativa during dehydration. Plant Physiol 99: 488–494
Bray EA (1991) Regulation of gene expression by endogenous ABA during drought stress. In: Davis WJ, Jones HG (eds) Abscisic acid physiology and biochemistry. BIOS Scientific Publishers Limited, Oxford, UK, pp 81–98
Chandler PM, Robertson M (1994) Gene expression regulated by abscisic acid and its relation to stress tolerance. Annu Rev Plant Mol Biol 45: 113–141
Chandler PM, Walker-Simmons M, King RW, Crouch M, Close TJ (1988) Expression of ABA-inducible genes in water stressed cereal seedlings. (Abstr 143) J Cell Biochem Suppl 12C
Close TJ, Kortt AA, Chandler PM (1989) A cDNA-based comparison of dehydration-induced proteins (dehydrins) in barley and corn. Plant Mol Biol 13: 95–108
Close TJ, Fenton RD, Moonan F (1993a) A view of plant dehydrins using antibodies specific to the carboxy terminal peptide. Plant Mol Biol 23: 275–286
Close TJ, Fenton RD, Yang A, Asghar R, DeMason DA, Crone DE, Meyer NC, Monnan F (1993b) Dehydrin: The protein. In: Close TJ, Bray EA (eds) Responses of plants to cellular dehydration during environmental stress. American Society of Plant Physiologists, Rockville, MD, pp 104–118
Dure LS, III (1985) Embryogenesis and gene expression during seed formation. Oxford Surv Plant Mol Cell Biol 2: 179–188
Dure LS, III (1993) The Lea proteins of higher plants. In: Verma DPS (ed) Control of plant gene expression. CRC Press, Inc, Boca Raton, Florida, pp 325–335
Dure LS, III, Crouch M, Harada J, Ho T-HD, Mundy J, Quatrano R, Thomas T, Sung ZR (1989) Common amino acid sequence domains among the LEA proteins of higher plants. Plant Mol Biol 12: 475–486
Espelund M, Saeboe-Larssen S, Hughes DW, Galan GA, Larsen F, Jakobsen KS (1992) Late embryogenesis-abundant genes encoding proteins with different numbers of hydrophilic repeats are regulated differentially by abscisic acid and osmotic stress. Plant J 2: 241–252
Galau GA, Close TJ (1992) Sequences of the cotton group 2 LEA/RAB/dehydrin proteins encoded byLea 3 cDNAs. Plant Physiol 98: 1523–1525
Galau GA, Hughes DW (1987) Coordinate accumulation of homologous transcripts of seven cottonLea gene families during embryogenesis and germination. Dev Biol 123: 213–221
Galau GA, Chlan CA, Dure L, III (1983) Developmental biochemistry of cottonseed embryogenesis and germination. XVI. Analysis of the principal cotton storage protein gene family with cloned cDNA probes. Plant Mol Biol 2: 189–198
Galau GA, Hughes DW, Dare LS, III (1986) Abscisic acid induction of cloned cotton late embryogenesis-abundant (Lea) mRNAs. Plant Mol Biol 7: 155–170
Galau GA, Bijaisoradat N, Hughes DW (1987) Accumulation kinetics of cotton late embryogenesis-abundant mRNAs: coordinate regulation during embryogenesis and the role of abscisic acid. Dev Biol 123: 198–212
Galau GA, Jakobsen KS, Hughes DW (1991) The controls of late dicot embryogenesis and early germination. Physiol Plant 81: 280–288
Gomez J, Sánchez-Martinez D, Stiefel V, Rigau J, Puigdomènech P, Pagès M (1988) A gene induced by the plant hormone abscisic acid in response to water stress encodes a glycine-rich protein. Nature 334: 262–264
Greenwood JS, Bewley JD (1982) Seed development inRicinus communis (castor bean). I. Descriptive morphology. Can J Bot 60: 1751–1760
Harada JJ, DeLisle AJ, Baden CS, Crouch ML (1989) Unusual sequence of an abscisic acid-inducible mRNA which accumulates late in Brassica napus seed development. Plant Mol Biol 12: 395–401
Hughes DW, Galan GA (1987) Translation efficiency ofLea mRNAs in cotton embryos: minor changes during embryogenesis and germination. Plant Mol Biol 9: 301–313
Hughes DW, Galan GA (1989) Temporally modular gene expression during cotyledon development. Genes Dev 3: 358–369
Jacobsen JV, Shaw DC (1989). Heat-stable proteins and abscisic acid action in barley aleurone cells. Plant Physiol 91: 1520–1526
Jiang L, Downing W, Baszczynski C, Kermode AR (1995) The 5′ flanking regions of vicilin and napin storage protein genes are down-regulated by desiccation in transgenic tobacco. Plant Physiol 107: 1439–1449
Kermode AR (1990) Regulatory mechanisms involved in the transition from seed development to germination. Crit Rev Plant Sci 9: 155–195
Kermode AR (1995) Regulatory mechanisms in the transition from seed development to germination: Interactions between the embryo and the seed environment. In: Galili G, Kigel J (eds) Seed development and germination. Marcel Dekker, Inc., New York, pp 273–332
Kermode AR, Bewley JD (1985a) The role of maturation drying in the transition from seed development to germination. I. Acquisition of desiccation-tolerance and germinability during development ofRicinus communis L. seeds. J Exp Bot 36: 1906–1915
Kermode AR, Bewley JD (1985b) The role of maturation drying in the transition from seed development to germination. II. Postgerminative enzyme production and soluble protein synthetic pattern changes within the endosperm ofRicinus communis L. seeds. J Exp Bot 36: 1916–1927
Kermode AR, Gifford DJ, Bewley JD (1985) The role of maturation drying in the transition from seed development to germination. III. Insoluble protein synthetic pattern changes within the endosperm ofRicinus communis L. seeds. J Exp Bot 36: 1928–1936
Kermode AR, Pramanik SK, Bewley JD (1989) The role of maturation drying in the transition from seed development to germination. VI. Desiccation-induced changes in messenger RNA populations within the endosperm ofRicinus communis L. seeds. J Exp Bot 40: 33–41
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685
Mundy J, Chua N-H (1988) Abscisic acid and water-stress induce the expression of a novel rice gene. EMBO J 7: 2279–2286
Pagés M, Vilardell J, Jensen AB, Alba MM, Torrent M, Goday A (1995) Molecular biological responses to drought in maize. In: Jackson M, Black C (eds) Interacting stresses on plants in a changing climate. NATO/ASI, Springer-Verlag, Berlin
Raynal M, Depigny D, Cooke R, Delseny M (1989) Characterization of a radish nuclear gene expressed during late seed maturation. Plant Physiol 91: 829–836
Robertson M, Chandler PM (1992) Pea dehydrins: identification, characterization and expression. Plant Mol Biol 19: 1031–1044
Skriver K, Mundy J (1990) Gene expression in response to abscisic acid and osmotic stress. Plant Cell 2: 503–512
Thomas TL, Vivekananda J, Bogue MA (1991) ABA regulation of gene expression in embryos and mature plants. In: Davies WJ, Jones HG (eds) Abscisic acid physiology and biochemistry. BIOS Scientific Publishers Ltd, Oxford, UK, pp 125–135
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Han, B., Wayne Hughes, D., Galau, G.A. et al. Changes in late-embryogenesis-abundant (LEA) messenger RNAs and dehydrins during maturation and premature drying ofRicinus communis L. seeds. Planta 201, 27–35 (1997). https://doi.org/10.1007/BF01258677
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01258677