Summary
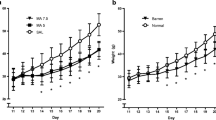
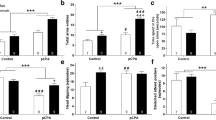
The aim of the study was to examine the effects of early postnatal exposure to nomifensine, an inhibitor of catecholamine uptake, on concurrent active (REM) sleep, on later alcohol related behavior and on monoamine concentrations in various brain regions of rats. For these purposes rats were given daily injections of 10 mg/kg nomifensine s.c. between the 7th and the 18th postnatal days. During the nomifensine exposure active sleep, expressed as a percentage of total sleeping time, was reduced. At one month of age, the nomifensine rats showed increased ambulation and had lower defecation scores in the open-field than the controls. Neonatal exposure to nomifensine increased voluntary intake of 10% (v/v) alcohol when the rats were 2–3 months of age. The rats, however, did not exhibit perseveration in the T-maze, and similarly to control rats suppressed drinking 0.1 M lithium chloride even when thirsty. Measurement of cerebral monoamine concentrations at the age of 3 months suggested that neonatal nomifensine treatment interferes with the noradrenergic and serotonergic systems in several regions of the brain. Concentrations of noradrenaline and 5-hydroxy-indoleacetic acid (5-HIAA) were decreased in the cerebral cortex and frontal cortex, concentration of 5-HIAA was decreased in the neostriatum, and concentrations of noradrenaline, 5-hydroxytryptamine (5-HT) and 5-HIAA were elevated in the lower brain stem. Taken together, these findings show that exposure to nomifensine during the 2nd and 3rd postnatal weeks suppresses neonatal active sleep, causes changes in the adult open-field behavior, and increases voluntary alcohol intake, perhaps due to a long-lasting alteration in brain monoamines.
Similar content being viewed by others
References
Adrien J (1981) Monoaminergic regulation of sleep: development and plasticity. In: Monset-Couchard M, Minkowski A (eds) Physiological and biochemical basis for perinatal medicine. Karger, Basel, pp 296–307
Alihanka J, Vaahtoranta K, Saarikivi J (1981) A new method of long-term monitoring of the ballistocardiogram, heart rate and respiration. Am J Physiol 240: 384–392
Broitman ST, Donoso AO (1978) Effects of chronic imipramine and clomipramine oral administration on maternal behavior and litter development. Psychopharmacology 56: 93–101
Carlsson A, Lindqvist M (1973) Effect of ethanol on the hydroxylation of tyrosine and tryptophan in rat brain in vivo. J Pharm Pharmacol 25: 437–444
Costell B, Kelly DM, Naylor RJ (1975) Nomifensine: a potent dopaminergic agonist of antiparkinson potential. Psychopharmacologia (Berlin) 41: 153–164
Coyle IR (1975) Changes in developing behavior following prenatal administration of imipramine. Pharmacol Biochem Behav 3: 799–807
Coyle JT (1977) Biochemical aspects of neurotransmission in the developing brain. In: Smythies JR, Bradley RJ (eds) International review of neurobiology, vol 20. Academic Press, New York, pp 65–103
Coyle JT (1982) Development of neurotransmitters in the neocortex. In: Rakic P, Goldman-Rakic PS (eds) Development and modifiability of the cerebral cortex. The MIT Press Journals, Massachusetts, pp 479–491
Cuomo V, Cortese I, Cagiano R, Renna G, Racagni G (1984) Behavioral changes in rats after prenatal administration of typical and atypical antidepressants. In: Chambers PL, Preziosi P, Chambers CM (eds) Disease, metabolism and reproduction in the toxic response to drugs and other chemicals. Arch Toxicol [Suppl] 7: 504–507
Daoust M, Saligut C, Chadelaud M, Chretien P, Moore N, Boismare F (1984) Attenuation by antidepressant drugs of alcohol intake in rats. Alcohol 1: 379–383
De Ceballos ML, Benedi A, Urdin C, Del Rio J (1985) Prenatal exposure of rats to antidepressant drugs down-regulates beta-adrenoceptors and 5-HT 2 receptors in cerebral cortex. Neuropharmacology 24: 947–952
Depoortere H (1983) Etudes electroencephalographiques des antidepresseurs. Assoc Franc Psych Biol 22: 252–253
Drago F, Continella G, Alloro MC, Scapagnini U (1985) Behavioral effects of perinatal administration of antidepressant drugs in the rat. Neurobehav Toxicol Teratol 7: 493–497
Egger G, Livesey P, Dawson R (1973) Ontogenetic aspects of central cholinergic involvement in spontaneous alternation behavior. Dev Psychobiol 6: 289–299
Eriksson K (1969) Factors affecting voluntary alcohol consumption in the albino rat. Ann Zool Fenn 6: 227–265
Erkinjuntti M, Vaahtoranta K, Alihanka J, Kero P (1984) Use of the SCSB method for monitoring of respiration, body movements and ballistocardiogram in infants. Early Human Dev 9: 119–126
File SE, Tucker JC (1983) Prenatal treatment with clomipramine has an anxiolytic profile in the adolescent rat. Physiol Behav 31: 57–61
Haikala H (1986) Different changes in striatal dopamine metabolism induced by nicotine in mice kept at different ambient temperatures. Evidence for partly separate metabolic routes of dopamine derived from separate compartmentations. Naunyn Schmiedebergs Arch Pharmacol 334: 373–376
Hallman H, Jonsson G (1984) Neurochemical studies on central dopamine Neurons—regional characterization of dopamine turnover. Med Biol 62: 198–209
Hilakivi LA, Hilakivi I (1986) Sleep-wake recordings in newborn rats by using a movement sensitive method. Behav Brain Res 19: 241–248
Hilakivi LA, Sinclair JD, Hilakivi I (1984) Effects of neonatal treatment with clomipramine on adult ethanol related behavior in the rat. Dev Brain Res 15: 129–132
Hilakivi LA, Stenberg D, Sinclair JD, Kiianmaa K (1987) Neonatal desipramine or zimeldine treatment causes long-lasting changes in brain monoaminergic systems and alcohol related behaviors in rats. Psychopharmacology 91: 403–409
Hunt P, Kannengiesser MH, Raynaud JP (1974) Nomifensine: A new potent inhibitor of dopamine uptake into synaptosomes from rat brain corpus striatum. J Pharm Pharmacol 26: 370–371
Jouvet M (1980) Paradoxical sleep and nature-nurture controversy. In: Connell P et al. (eds) Progress in brain research, vol 53. Adaptive capabilities of the nervous system. Elsevier, Amsterdam, pp 331–346
Jouvet-Mounier, D, Astic L, Laconte D (1970) Ontogenesis of the stages of sleep in rat, cat and guinea pig during the first postnatal month. Dev Psychobiol 2: 216–239
Kiianmaa K, Attila LMJ (1979) Alcohol intake, ethanol-induced narcosis and intoxication in rats following neonatal 6-hydroxydopamine or 5, 7-dihydroxytryptamine treatment. Naunyn Schmiedebergs Arch Pharmacol 308: 165–170
Lelkes Z, Obal F Jr, Benedek G, Rubicsek G, Alföldi P (1987) Effects of an antidepressant, nomifensine, on sleep in rats. Naunyn Schmiedebergs Arch Pharmacol, in press
Mabry PD, Campbell BA (1977) Developmental psychopharmacology. In: Iversen LL et al (eds) Principles of behavioral pharmacology 7. Handbook of Psychopharmacology, vol 2. Plenum Press, New York, pp 393–444
Mirmiran M, Van de Poll NE, Corner MA, Oyen HG, Bour HL (1981) Suppression of active sleep by chronic treatment with clomipramine during postnatal development: Effects upon adult sleep and behavior in the rat. Brain Res 204: 129–146
Mirmiran M, Scholtens J, Van de Poll NE, Uylings HB, Van der Gugten G, Boer GJ (1983) Effects of experimental suppression of active (REM) sleep during early development upon adult brain and behavior in the rat. Dev Brain Res 7: 227–286
Ponzio F, Jonsson G (1978) Effects of neonatal 5, 7-dihydroxytryptamine treatment on the development of serotonin neurons and their transmitter metabolism. Dev Neurosci 1: 80–89
Riley EP, Lochry EA, Shapiro NR (1979 a) Lack of response inhibition in rats prenatally exposed to alcohol. Psychopharmacology 62: 47–62
Riley EP, Lochry EA, Shapiro NR, Baldwin J (1979 b) Response perseveration in rats exposed to alcohol prenatally. Pharmacol Biochem Behav 10: 225–259
Roffwarg HP, Muzio JN, Dement WC (1966) Ontogenetic development of the human sleep-dream cycle. Science 52: 604–619
Sachs C, Jonsson G (1975) Changes in the development of central noradrenaline neurons following 6-OH-DA administration. In: Jonsson G, Malmfors T, Sachs Ch (eds) Chemical tools in catecholamine research, vol 1. North-Holland, New York, pp 163–171
Schacht U, Heptner W (1974) Effect of nomifensine (HOE 984), a new antidepressant, on uptake of noradrenaline and serotonin and on release of noradrenaline in rat brain synaptosomes. Biochem Pharmacol 23: 3143–3422
Scherschlicht R, Polc P, Schneeberger J, Steiner M, Haefely W (1982) Selective suppression of rapid eye movement sleep (REMS) in cats by typical and atypical antidepressants. In: Costa E, Racagni G (eds) Typical and atypical antidepressants: molecular mechanisms. Raven Press, New York, pp 359–364
Shaywitz BA, Teicher MH, Cohen DJ, Anderson GM, Young JG, Levitt P (1984) Dopaminergic but not noradrenergic mediation of hyperactivity and performance deficits in the developing rat pup. Psychopharmacology 82: 73–77
Shaywitz BA, Yager RD, Klopper JH (1976) Selective brain dopamine depletion in developing rats: an experimental model of minimal brain dysfunction. Science 191: 305–308
Tissari A (1975) Pharmacological and ultrastructural maturation of serotonergic synapses during ontogeny. Med Biol 53: 1–14
Walsh RN, Cummins RA (1976) The open-field test: a critical review. Psychol Bull 83: 482–504
Westerink BHC, Mulder TBA (1981) Determination of picomole amounts of dopamine, noradrenaline, 3, 4-dihydroxyphenylalanine, 3, 4-dihydroxyphenylacetic acid, homovanillic acid, and 5-hydroxyindolacetic acid in nervous tissue after one-step purification on Sephadex G-10, using high-performance liquid chromatography with a novel type of electrochemical detection. J Neurochem 36: 1449–1462
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hilakivi, L.A., Hilakivi, I., Ahtee, L. et al. Effect of neonatal nomifensine exposure on adult behavior and brain monoamines in rats. J. Neural Transmission 70, 99–116 (1987). https://doi.org/10.1007/BF01252512
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01252512