Abstract
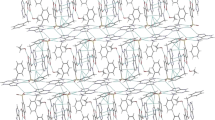
Crystals of 9-methyladenine salicylate (C6H8N +5 ·C7H5O −3 ), mp 126°C, are orthorhombic, space groupPbcn (D 142h , No. 60) witha=25.305(3),b=8.066(1),c=12.928(2) Å,d c=1.446 g cm−3,d m=1.43 g cm−3 (CCl4/ cyclohexane) forZ=8. The 9-methyladenine, protonated at N(1), and salicylate form a pair of strong hydrogen bonds with N⋯O distances of 2.617 and 2.858 Å. In addition, a hydrogen bond is formed between two adenine moieties using the amino group and imidazole nitrogen, N(7), with N⋯N separation of 3.114 Å. As in salicylic acid, an intermolecular hydrogen bond of 2.560 Å is formed between the hydroxyl and a carboxyl oxygen atoms.
Similar content being viewed by others
References
Cassay, R. E., and Hawkinson, S. W. (1982)Acta Cryst. B 38, 2206.
Cochran, W. (1951)Acta Cryst. 4, 81.
Donbrow, M., and Jan, Z. A. (1965)J. Pharm. Pharmacol. Suppl. 17, 1295.
Eckert, T. (1962)Arch. Pharm. 295, 233.
Hamilton, W. C. (1963) InStructural Chemistry and Molecular Biology, A. Rich and N. Davidson, eds. (Freeman, San Francisco) p. 468.
Higuchi, T., and Lach, J. L. (1954)J. Am. Pharm. Assoc. Sci. Ed. 43, 349.
Higuchi, T., and Zuck, Z. A. (1952)J. Am. Pharm. Assoc. Sci. Ed. 41, 10.
Higuchi, T., and Zuck, Z. A. (1953)J. Am. Pharm. Assoc. Sci. Ed. 42, 132.
Kim, H. S., and Jeffrey, G. A. (1971)Acta Cryst. B 27, 1123.
Kraut, J., and Jensen, L. H. (1963)Acta Cryst. 16, 79.
Marsh, R. E., and Donohue, J. (1967)Adv. Protein Chem. 22, 239.
Main, P. (1980) Natl. Resour. Comput. Chem. Software Cat., Vol. 1, Prog. No. XS01 (Multan 78).
Ramakrishnan, C., and Prasad, N. (1971)J. Protein Res. 3, 209.
Rich, A., Davies, D. R., Crick, F. H. C., and Watson, J. D. (1961)J. Mol. Biol. 3, 71.
Shefter, E. (1968)J. Pharm. Sci. 57, 1163.
Shefter, E. (1969)J. Pharm. Sci. 58, 710.
Shefter, E., Barlow, M., Sparks, R. A., and Trueblood, K. N. (1969)Acta Cryst. B 25, 895.
Shefter, E., Brennan, T. F., and Sackman, P. (1971)Chem. Pharm. Bull. 19, 746.
Sletten, J., and Jensen, L. H. (1969)Acta Cryst., B 25, 1608.
Sundaralingam, M. (1966)Acta Cryst. 21, 495.
Sundaralingam M., and Jensen, L. H. (1965)Acta Cryst. 18, 1053.
Voet, D., and Rich, A. (1970).Prog. Nucleic Acid Res. Mol. Biol. 10, 183.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gellert, R.W., Hsu, IN. The structure of 9-methyladenine salicylate. Journal of Crystallographic and Spectroscopic Research 13, 99–105 (1983). https://doi.org/10.1007/BF01246579
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01246579