Abstract
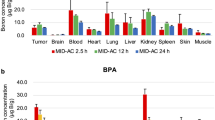
We described previously that10B atoms delivered by monoclonal antibody (mAb) exerted a cytotoxic effect on AH66 cells in a dose-dependent manner upon thermal neutron irradiationin vitro. In the present study, the delivering capacity of boronated anti-(α-fetoprotein) (AFP) mAb to carry10B atoms to AFP-producing tumor xenografts in nude mice was determined. Boronated mAb was prepared by conjugating 50 mM 10B compound to an anti-AFP mAb (2 mg/ml) usingN-succinimidyl-3-) (2-pyridyldithio) propionate. The number of10B atoms conjugated directly to the mAb was estimated to be 459/antibody by prompt γ-ray spectrometry. Boron concentrations in tumor tissue obtained 12, 24, 72, and 120 h after injection of 3.0 mg10B-conjugated anti-AFP mAb were 11.10±3.12 (SD,n=6), 29.30±5.11, 33.02±11.8, and 12.91±5.62 ppm respectively. For control10B-conjugated anti-dinitrophenol (DNP) mAb, the values were 9.59±0.99, 10.37±2.86, 10.00±2.95, and 8.83±4.71 ppm respectively. The concentrations in blood were less than 0.40±0.10 ppm with anti-AFP mAb and less than 0.51±0.15 ppm with anti-DNP mAb at each sampling time (12, 24, 72, and 120 h). The number of10B atoms delivered to the tumor cells was calculated to be 0.62×109, 1.63×109, 1.84×109 and 0.72×109 at each sampling time after injection of10B-anti-AFP mAb. The amount of10B atoms necessary for effective boron neutron-capture therapy was estimated to be 109/tumor cell. We were able to carry 1.84×109 10B atoms to AH66 tumor cells by using10B-anti-AFP mAb. The accumulation reached its peak 72 h after injection. These data indicated that the10B-conjugated antitumor mAb could deliver a sufficient amount of10B atoms to the tumor cells to induce cytotoxic effects 72 h after injection upon thermal neutron irradiation.
Similar content being viewed by others
Abbreviations
- BNCT:
-
boron neutron-capture therapy
- AFP:
-
α-fetoprotein
- SPDP:
-
N-succinimidyl-3-(2-pyridyldithia) propionate
- DNP:
-
dinitrophenol
References
Alam F, Soloway AH, Barth RF (1984) Boronation of polyclonal and monoclonal antibodies for neutron capture. Brookhaven Natl Lab Rep. 51730:229–236
Alam F, Soloway AH, McGuire JE (1985) DicesiumN-succinimidyl-3-(undecahydro-closo-dodecaboranyldithio) propionate, a novel hetero-bifunctional boronating agent. J Med Chem 28:522–525
Barth RF, Johnson CW, Wei WZ, Carey WE, Soloway AH, McGuire J (1982) Neutron capture using boronated monoclonal antibody directed against tumor-associated antigens. Cancer Detect Prev 5:315–323
Barth RF, Alam F, Soloway AH, Adams DM, Steplewski Z (1986) boronated monoclonal antibody 17-1A for potential neutron capture therapy of colorectal cancer. Hybridoma 5:43–50
Coderre J, Glass JD, Fairchild RG, Roy U, Cohen S, Fand I (1987) Selective targeting of boronophenylalanine to melanoma in BALB/c mice for neutron capture therapy. Cancer Res 47:6377–6383
Ey PL, Prowse SJ, Jenhin CR (1978) Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-Sepharose. Immunochemistry 15:429–436
Fairchild RG, Bond VP (1985) Current status of10B-Neutron capture therapy: enhancement of tumor dose via beam filtration and dose rate, and the effects of these parameters on minimum boron content: a theoretical evaluation. Int J Radiat Oncol Biol Phys 11:831–840
Gabel D, Holstein H, Lasson B, Gille L, Ericson G, Sacker D, Som P Fairchild RG (1987) Quantitative neutron capture radiography for studying the biodistribution of tumor-seeking boron-containing compounds. Cancer Res 47:5451–5454
Goldenberg DM, Sharkey RM, Primus FJ, Mizusawa E, Hawthorne ME (1984) Neutron-capture therapy of human cancer:in vivo results on tumor localization of boron-10 labeled antibodies to carcinoembryonic antigen in the GW-39 tumor model system. Proc Natl Acad Sci USA 81:560–563
Hatanaka H (1986) Clinical experience of boron neutron capture therapy for gliomas — a comparison with conventional chemo-immuno-radiotherapy. In: Boron-neutron capture therapy for tumors. Nishimura, Niigata, pp 349–378
Isaka H, Umehara S, Yoshii H, Tsukada Y, Hiraii H (1976) α-Fetoprotein and albumin produced by subclonal cell population of the ascites hepatoma AH66in vitro. Gann 67:131–135
Kobayashi T, Kanda K (1983) Microanalysis system or ppm-order10B concentrations in tissue for neutron capture therapy by prompt gamma-ray spectrometry. Nucl Instrum Methods 204:525–531
Kruger PC (1940) Some biological effects of nuclear disintegration products on neoplastic tissue. Proc Natl Acad Sci USA 26:181–192
Leserman LD, Machy P, Barbet J (1981) Cell-specific drug transfer from liposomes bearing monoclonal antibodies. Nature 293:226–228
Locher GL (1936) Biological effects and therapeutic possibilities of neutrons. Am J Roentgenol Radium Ther 36:1–13
Mishima Y, Ichihashi M, Nakanishi T, Tsiyi M, Ueda M, Nakagawa T, Suzuki T (1983) Cure of malignant melonamo by single thermal neutron capture treatment using melanoma-seeking compounds. Proceedings of First International Symposium on Neutron Capture Therapy. Brookhaven Natl Lab Rep 51730:355–364
Mishima Y, Ichihashi M, Tsuji M, Ueda M, Hatta S, Nakagawa T, Tanaka C, Taniyama K (1986) Prerequisites of first clinical trial for melanoma selective thermal neutron capture therapy: In: Neutron capture therapy. Proceedings of the Second International Symposium on Neutron Capture Therapy. Nishimura, Niigata, pp 230–236
Mishima Y, Honda C, Ichihashi M (1989) Treatment of malignant melanoma by single thermal neutron capture therapy with melanomaseeking 10B-compound. Lancet II:388–389
Seldinger SI (1953) Catheter replacement of the needle in percutaneous arteriography. A new technique. Acta Radiol 39:368–376
Takahashi T, Fujii Y, Fujii G, Nariuchi H (1987) Preliminary study for application of anti-alpha-fetoprotein monoclonal antibody to boronneutron capture therapy. Jpn J Exp Med 57:83–91
Tsukada Y, Bischof WKD, Hibi H (1982) Effect of a conjugate daunomycin and antibodies to rat α-fetoprotein on the growth of α-fetoprotein-producing tumor cells. Proc Natl Acad Sci USA 79:621–625
Umeda M, Ishimori Y, Yoshikawa K, Takada M, Yasuda T (1986) Homogeneous determination of C-reactive protein in serum using liposome immune lysis assay (LILA). Jpn J Exp Med 56:35–42
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yanagië, H., Fujii, Y., Sekiguchi, M. et al. A targeting model of boron neutron-capture therapy to hepatoma cellsin vivo with a boronated anti-(α-fetoprotein) monoclonal antibody. J Cancer Res Clin Oncol 120, 636–640 (1994). https://doi.org/10.1007/BF01245373
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01245373