Abstract
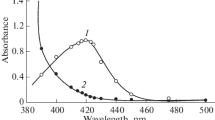
A simple method is described for the rapid spectrophotometric determination of molybedenum in synthetic and industrial samples containing 0.1-5% Mo. Molybdenum is reduced with ascorbic acid at room temperature in 1 mol dm−3 H2SO4 and extracted with chloroform after adding 2-(2′-furyl)-3-hydroxychromone (FHC). The yellow colour of the 1 ∶ 2 Mo-FHC complex is measured at 414 nm against a reagent blank. Beer's law is obeyed over the range 0-2.7 μg Mo cm−3 of solvent phase. The molar absorptivity and Sandell's sensitivity are 5.18 × 104 dm3 mol−1 cm−1 and 0.0018 μg Mo cm−2, respectively. Relative standard deviations are 0.2% for solutions and 0.5–1.5% for solid samples. Interference from tungsten and tin is removed by adding citrate and EDTA, respectively, while niobium and tantalum are masked by fluoride. Many elements such as V, Fe, Ti, U, Mn, Cr, Co, Ni, Re, Ru, Pt, Rh, Se, Au, Bi, Zr, Th, Ce, As and Al do not interfere even in large amounts, but antimony always interferes. Among the anions and complexing agents, only thiocyanate interferes seriously.
Similar content being viewed by others
References
S. Rubio-Barroso, L. M. Polo-Diez,An. Quim., Ser. B 1987,83, 67.
Gy. Almassy, M. Vigvari,Magy. Kem. Foly. 1956,62, 332.
K. Yamamoto, J. Hara, K. Ohashi,Anal. Chim. Acta 1982,135, 173.
F. L. Chan, R. W. Moshier,Talanta 1960,3, 272.
G. Goldstein, D. L. Manning, O. Menis,Anal. Chem. 1958,30, 539.
K. Kodama,Methods of Quantitative Inorganic Analysis, Interscience, New York, 1963, p. 216.
A. Carvaiser,Bull. Soc. Chim., Fr. 1962, 528.
A. I. Busev,Analytical Chemistry of Molybdenum, Program for Scientific Translations, Jerusalem, 1964, p. 108.
P. Bermejo Barrera, J. F. Vazquez Gonzalex, F. Bermejo Martinex,Microchem. J.,1987,35, 1.
A. Ringbom,Fresenius Z. Anal. Chem. 1938,115, 332.
P. Job,Ann. Chim. Paris 1928,9, 113.
W. C. Vosburg, G. R. Cooper,J. Am. Chem. Soc. 1941,63, 437.
J. H. Yoe, A. L. Jones,Ind. Eng. Chem., Anal. Edn. 1944,16, 111.
H. Onishi, in:Chemical Analysis, Vol. 3, Part IIB, Wiley, New York, 1989, Table 25-2.
Z. Marczenko,Spectrophotometric Determination of Elements, Wiley, New York, 1976, pp. 360–365.
M. R. P. Reddy, P. V. S. Kumar, J. P. Shyamsunder, Y. Anjaneyulu,Proc. Indian Natl. Sci. Acad., Part A,1990,56, 255.
M. Gao, F. Zhang, X. Liang,Yejin Fenxi,1991,11, 48.
I. Baranowska, K. Barszczewska,Talanta,1992,39, 1205.
F. D. Snell,Photometric and Fluorometric Methods of Analysis: Metals, Part 2, Wiley, New York, 1978, pp. 1295–1349.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dass, R., Mehta, J.R. Spectrophotometric determination of molybdenum(V) by extraction of its 2-(2′-furyl)-3-hydroxychromone complex. Mikrochim Acta 113, 37–43 (1994). https://doi.org/10.1007/BF01243135
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01243135