Summary
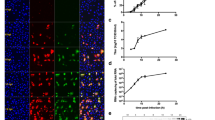
Based on the detection of viral antigen by means of immunofluorescence staining, the replication cycle of parvovirus Lu III was studied in randomly growing and synchronously multiplying HeLa cells. Two different processes could be distinguished in both culture systems. Early events consisted in the appearance of virus specific antigen 3–4 hours following infection independend on the actual physiologic state of the infected cell. The development of intranuclear fluorescence, however, started as early as 8–10 hours p.i. and proved strictly bound to cellular events. Viral replication was finished 16–18 hours p.i. when brightly staining nuclei released distinct fluorescing granules into the cytoplasm or were lost from the cell sheet.
Infection of synchronized HeLa cells at different points of the cell cycle revealed that the shortest possible synchronous replication of Lu III virus could be obtained only if infection occurred about 3 hours after beginning of S phase. Since viral adsorption, penetration, uncoating, as well as transcription of early antigen are independent on cellular physiology and take at least 3 hours, the status of “physiologic competence” necessary for successful completion of virus multiplication may be identical with events in late S phase.
Finally, experiments with UV-inactivated virus suspensions pointed to the presence of a factor which is unaffected by UV irradiation and interferes temporarely with cellular DNA synthesis.
Similar content being viewed by others
References
Al-Lami, F., N. Ledinko, andH. Toolan: Electron microscope study of human NB and SMH cells infected with the parvovirus H-1: Involvement of the nucleolus. J. gen. Virol.5, 485–492 (1969).
Bernhard, W., F. H. Kasten etCh. Chany: Étude cytochimique et ultrastructurale de cellules infectées par le virus K du rat et le virus H-1. C. R. Acad. Sci. (Paris)257, 1566–1569 (1963).
Cole G. A., andN. Nathanson: Immunofluorescent studies of the replication of rat virus (HER strain) in tissue culture. Acta virol.13, 515–520 (1969).
Fields, H. A., andB. L. Nicholson: The replication of Kilham rat virus (RV) in various host systems: immunofluorescent studies. Canad. J. Microbiol.18, 103–107 (1972).
Hallauer, C., G. Kronauer, andG. Siegl: Parvoviruses as contaminants of permanent human cell lines. I. Virus isolations from 1960–1970. Arch. ges. Virusforsch.35, 80–90 (1971).
Hallauer, C., G. Siegl, andG. Kronauer: Parvoviruses as contaminants of permanent human cell lines. III. The biologic properties of the isolated viruses. Arch. ges. Virusforsch.38, 366–382 (1972).
Hampton, E. G.: H-1 virus growth in synchronized rat embryo cells. Canad. J. Microbiol.16, 266–268 (1970).
Lesser, B., andT. P. Brent: Cold storage as a method for accumulating mitotic HeLa cells without impairing subsequent synchronous growth. Exp. Cell Res.62, 470–473 (1970).
Margolis, G., andL. Kilham: Rat virus, an agent with an affinity for the dividing cells. In: Slow, Latent and Temperate Virus Infections, National Institute of Neurological Diseases and Blindness Monograph No.2, pp. 361–367 (1965).
Mayor, H. D., andE. L. Jordan: Electron microscopic study of the rodent “Picodnavirus” X-14. Exp. molec. Path.5, 580–589 (1966).
Mayor, H. D., andM. Ito: The early detection of picodnavirus X-14 by immuno-fluorescence. Proc. Soc. exp. Biol. (N. Y.)129, 684–686 (1968).
Siegl, G., C. Hallauer, A. Novak, andG. Kronauer: Parvoviruses as contaminants of permanent human cell lines. II. Physicochemical properties of the isolated viruses. Arch. ges. Virusforsch.35, 91–103 (1971).
Siegl, G., C. Hallauer, andA. Novak: Parvoviruses as contaminants of permanent human cell lines. IV. Multiplication of KBSH virus in KB cells. Arch. ges. Virusforsch.36, 351–362 (1972).
Tennant, R. W., K. R. Layman, andR. E. Hand, Jr.: Effect fo cell physiological state on infection by rat virus. J. Virol.4, 872–878 (1969).
Tennant, R. W., andR. E. Hand, Jr.: Requirement of cellular synthesis for Kilham rat virus replication. Virology42, 1054–1063 (1970).
Tennant, R. W.: Inhibition of mitosis and macromolecular synthesis in rat embryo cells by Kilham rat virus. J. Virol.8, 402–408 (1971).
Terasima, T., andL. J. Tolmach: Growth and nucleic acid synthesis in synchronously dividing populations of HeLa cells. Exp. Cell Res.30, 344–362 (1963).
Yamashita, T., Y. Moritsugu, andH. Shimojo: Suppression of cellular DNA synthesis in growing cells by ultraviolet-irradiated adenoviruses. Virology45, 687–696 (1971).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Siegl, G., Gautschi, M. The multiplication of parvovirus Lu III in a synchronized culture system. Archiv f Virusforschung 40, 105–118 (1973). https://doi.org/10.1007/BF01242642
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01242642