Abstract
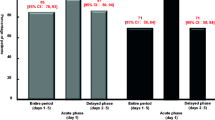
Twenty-nine patients with gynecologic malignancies were treated with a fixed low dose of intravenous ondansetron (8 mg) plus dexamethasone (20 mg) in an effort to develop an effective and less expensive antiemetic regimen for the control of carboplatin-induced emesis. Twenty-six (90%) of the women participating in this trial experienced complete control of both acute nausea and vomiting (developing within the first 24 h after chemotherapy administration), while 27 (93%) patients exhibited either complete or major control (≤2 episodes of vomiting, ≤5 episodes of retching, minimal interference with eating) of emesis. On the basis of our experience in this trial, we conclude that the combination of low dose (8 mg) intravenous ondansetron plus dexamethasone is a well-tolerated and highly cost-effective antiemetic strategy for individuals receiving carboplatin-based chemotherapy.
Similar content being viewed by others
References
Alberts DA, Green S, Hannigan EV, O'Toole R, Stock-Novack D, Anderson P, Surwit EA, Malvlya VK, Nahhas WA, Jolles CJ (1992) Improved therapeutic index of carboplatin plus cyclophosphamide versus cisplatin plus cyclophosphamide: Final report by the Southwest Oncology Group of a phase III randomized trial in stages III and IV ovarian cancer. J Clin Oncol 10: 706–717
Calvert AH, Harland SJ, Newell DR, Siddik ZH, Jones AC, McElwain TJ, Raju S, Wiltshaw E, Smith IE, Baker JM, Peckham MM, Harrap KR (1982) Early clinical studies withcis-diammine-1,1-cyclobutane dicarboxylate platinum II. Cancer Chemother Pharmacol 9: 140–147
Chapman SM, Pruemer JM (1993) Ondansetron use in a major university teaching hospital. Am J Hosp Pharm 50: 670–674
DeMulder PHM, Seynaeve C, Vermorken JB, van Liessum PA, Mols-Jevdevic S, Allman EL, Beranek P, Verweij J (1990) Ondansetron compared with high-dose metoclopramide in prophylaxis of acute and delayed cisplatin-induced nausea and vomiting: a multicenter, randomized, crossover study. Ann Intern Med 133: 834–840
Grunberg SM, Hesketh PJ (1993) Control of chemotherapy-induced emesis. N Engl J Med 329: 1790–1796
Harvey VJ, Evans BD, Mitchell PLR, Mak D, Neave LM, Langley GB, Dickson DSP (1991) Reduction of carboplatin induced emesis by ondansetron. Br J Cancer 63: 942–944
Hesketh PJ, Harvey WH, Harker WG, Beck TM, Ryan T, Bricker LJ, Kish JA, Murphy WK, Hainsworth JD, Haley B, Plagge P, Flack NE (1994) A randomized, double-blind comparison of intravenous ondansetron alone and in combination with intravenous dexamethasone in the prevention of high-dose cisplatin-induced emesis. J Clin Oncol 12: 596–600
Markman M, Kennedy A, Webster K, Kulp B, Peterson G, Belinson J (1996) Control of carboplatin-induced emesis with a fixed low-dose of granisetron (0.5 mg) plus dexamethasone. Gynecol Oncol 60: 435–437
Martin M, Diaz-Rubio E, Sanchez A, Almenarez J, Lopez-Vega JM (1990) The natural course of emesis after carboplatin treatment. Acta Oncologica 29: 593–595
Navari R, Gandara D, Hesketh P, Hall S, Mailliard J, Ritter H, Friedman C, Fitts D, on behalf of the Granisetron Study Group (1995) comparative clinical trial of granisetron and ondansetron in the prophylaxis of cisplatin-induced emesis. J Clin Oncol 13: 1242–1248
Perez EA (1995) Review of the preclinical pharmacology and comparative efficacy of 5-hydroxytryptamine-3 receptor antagonists for chemotherapy-induced emesis. J Clin Oncol 13: 1036–1043
Peters II MD, Long KS, Patel HS, Reitz JA, Jessen LM, Emhart GC (1993) Multicenter evaluation of ondansetron use in hospitalized oncology patients. Am J Hosp Pharm 50: 1164–1170
Rose WC, Schurig JE (1985) Preclinical antitumor and toxicologic profile of carboplatin, Cancer Treat Rev 12 (suppl A): 1–19
Smyth JF, Coleman RE, Nicolson M, Gallmeier WM, Leonard RCF, Cornbleet MA, Allan SG, Upadhyaya BK, Bruntsch U (1991) Does dexamethasone enhance control of acute cisplatin induced emesis by ondansetron? BMJ 303: 1423–1426
Swenerton K, Jeffrey J, Stuart G, Roy M, Krepart G, Carmichael J, Drouin P, Stanimir R, O'Connell G, MacLean G, Kirk ME, Canetta R, Koski B, Shelley W, Zee B, Pater J (1992) Cisplatin-cyclophosphamide versus carboplatin-cyclophosphamide in advanced ovarian cancer: A randomized phase III study of the National Cancer Institute of Canada Clinical Trials Groups, J Clin Oncol 10: 718–726
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Markman, M., Kennedy, A., Webster, K. et al. Low-dose intravenous ondansetron (8 mg) plus dexamethasone: an effective regimen for the control of carboplatin-induced emesis. J Cancer Res Clin Oncol 123, 224–226 (1997). https://doi.org/10.1007/BF01240319
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01240319