Summary
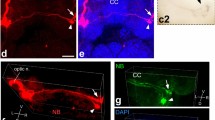
Like several other opisthobranch molluscs, the marine snailBulla gouldiana possesses two circadian pacemakers, one in each eye. The two ocular pacemakers are mutually coupled such that: (1) the circadian rhythms of spontaneous electrical activity recorded from the optic nerve are normally synchronous and; (2) if experimentally desynchronized the rhythms will return to the synchronized state. This coupling of the pacemakers is mediated by efferent fibers in the optic nerve, terminating in neuropil adjacent to the basal retinal neurons (BRNs), the putative circadian pacemaker cells. Attempts to identify neurotransmitters in efferent terminals that may be involved in the coupling process have failed. In the present study we demonstrate axons in the optic nerve and axon terminals adjacent to the BRNs that exhibit FMRF-amide- (molluscan cardioexcitatory peptide) and NPY-like (neuropeptide-Y) immunoreactivity. The pattern of immunoreactivity to both antisera is identical. Blocking studies indicate that both antisera are recognizing the same site, most likely the arginine-phenylalanine-amide terminus of FMRF, or an FMRF-like molecule. We conclude that FMRF is a candidate for the chemical mediator involved in the interaction between the two ocular pacemakers inBulla gouldiana.
Similar content being viewed by others
References
Block GD, McMahon DG, Wallace SF, Friesen WO (1984) Cellular analysis of theBulla ocular circadian pacemaker system. I. A model for retinal organization. J Comp Physiol A 155:365–378
Block GD, Yen L-H, Lusska AE (1985) Circadian pacemaker coupling inBulla: Efferent optic nerve impulses induce phase shifts in ocular pacemaker. Soc Neurosci [Abstr] 11:1138
Block GD, Friesen WO (1981) Electrophysiology ofBulla eyes: Circadian rhythm and intracellular responses to illumination. Soc Neurosci [Abstr] 7:45
Block GD, Roberts MH (1984) Circadian pacemaker coupling inBulla: Efferent impulses generate depolarizations in putative circadian pacemaker neurons. Soc Neurosci [Abstr] 10:292
Block GD, Wallace SF (1982) Localization of a circadian pacemaker in the eye of a mollusc,Bulla. Science 217:155–157
Card JP, Brecha N, Moore RY (1983) Immunohistochemical localization of avian pancreatic polypeptide-like immunoreactivity in the rat hypothalamus. J Comp Neurol 217:123–136
Corrent G, McAdoo DJ, Eskin A (1978) Serotonin shifts the phase of the circadian rhythm from theAplysia eye. Science 202:977–979
Gustafson EL, Card JP, Moore RY (1986) Neuropeptide Y localization in the rat amygdaloid complex. J Comp Neurol (in press)
Jacklet JW (1985) FMRFamide blockade of optic nerve pacemaker activity and FMRFamide immunoreactivity of optic nerve efferent fibers and retinal neuropil ofBulla andAplysia. Soc Neurosci [Abstr] 11:1139
Jacklet JW, Colquhoun W (1983) Ultrastructure of photoreceptors and circadian pacemaker neurons in the eye of a gastropod,Bulla. J Neurocytol 12:673–696
Luborsky-Moore JL, Jacklet JW (1976)Aplysia eye: Modulation by efferent optic nerve activity. Brain Res 115:501–505
Lundberg JM, Terenius L, Hökfelt T, Tatemoto K (1984) Comparative immunohistochemical and biochemical analysis of pancreatic polypeptide-like peptides with special reference to presence of neuropeptide Y in central and peripheral tissues. J Neurosci 4:2376–2386
McMahon DG, Block GD (1984) Circadian pacemaker entrainment inBulla: Light and serotonin affect the membrane potential of putative circadian pacemaker neurons. Soc Neurosci [Abstr] 10:292
McMahon DG, Block GD (1985) Intracellular current injection into putative circadian pacemaker neurons phase shifts theBulla ocular circadian pacemaker. Soc Neurosci [Abstr] 11:1138
McMahon DG, Wallace SF, Block GD (1984) Cellular analysis of theBulla ocular circadian pacemaker system II. Neurophysiological basis of circadian rhythmicity. J Comp Physiol A 155:379–385
Moore RY, Gustafson EL, Card JP (1984) Identical immunoreactivity of afferents to the rat suprachiasmatic nucleus with antisera against avian pancreatic polypeptide, molluscan cardioexcitatory peptide and neuropeptide Y. Cell Tissue Res 236:41–46
Nadakavukaren JJ, Lickey ME, Jordan WP (1986) Regulation of the circadian clock in theAplysia eye: Mimicry of neural action by serotonin. J Neurosci 6:14–21
Price DA, Greenberg MJ (1977) Structure of a molluscan cardioexcitatory neuropeptide. Science 197:670–671
Roberts MH, Block GD (1983) Mutual coupling between the ocular circadian pacemakers ofBulla gouldiana. Science 221:87–89
Roberts MH, Block GD (1986) Analysis of mutual circadian pacemaker coupling between the two eyes ofBulla. J Biol Rhythms 1:55–75
Ruben P, Johnson JW, Thompson S (1986) Analysis of FMRF-amide effects onAplysia bursting neurons. J Neurosci 6:252–259
Sternberger LA (1979) Immunohistochemistry (2nd ed.) Wiley, New York, pp 104–169
Takahashi JS, Eskin A (1985) Serotoninergic innervation of molluscan eyes containing circadian pacemakers. Soc Neurosci [Abstr] 11:537
Triepel J, Grimmelikhuijzen CPJ (1984) Mapping of neurons in the central nervous system of the guinea pig by use of antisera specific to the molluscan neuropeptide FMRF-amide. Cell Tissue Res 237:575–586
Weber E, Evans CJ, Samuelsson SJ, Brechas JD (1981) Novel peptide neuronal system in rat brain and pituitary. Science 214:1248–1251
Williams RG, Dockray GJ (1983) Immunohistochemical studies of FMRF-amide-like immunoreactivity in the rat brain. Brain Res 276:213–229
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Roberts, M.H., Moore, R.Y. Localization of neuropeptides in efferent terminals of the eye in the marine snail,Bulla gouldiana . Cell Tissue Res. 248, 67–73 (1987). https://doi.org/10.1007/BF01239964
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01239964