Abstract
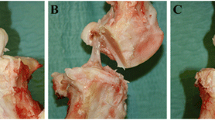
The midportion of the anterior cruciate ligament (ACL) of rabbits was partially transected, and the effect of hyaluronan (HA) on its healing was determined. A 1% solution of HA (HA group) or physiological phosphate-buffered saline (control group) was administered intraarticularly, at 0.1 ml/kg body weight, once a week from 1 week after the operation. Two, 4, and 6 weeks after the initiation of HA administration, the ACLs were examined grossly, histologically and immunohistochemically. At 2 weeks, the lacerated portions were completely covered with scar-like tissue in both groups. These tissue areas were smaller in the HA group than in the control group. Histologically in the HA group, the regularity of collagen fibers (indicating the maturity of regenerated collagen fibers) had increased compared to findings in the control group, and the number of fibroblastic cells decreased gradually at a significantly faster rate. The number of inflammatory cells and blood vessels decreased gradually in both groups, with these values being lower in the HA group at each time point but not significantly so. Immunohistochemical examination of the repaired tissue revealed strong staining with anti-chondroitin sulfate proteoglycan antibody in the HA group 2 weeks after the first HA administration. The staining gradually became reduced, with the rate of reduction being faster in the HA group than in the control group. The stimulation of chondroitin sulfate proteoglycan production and the faster reduction of it in the HA group suggests that HA facilitated tissue repair and inhibited the formation of scar tissue.
Similar content being viewed by others
References
Abatangelo G, Martelli M, Vecchia P. Healing of hyaluronic acid-enriched wounds: Histological observations. J Surg Res 1983;35: 410–6.
Akasaka H, Seto S, Yanagi M, et al. Industrial production of hyaluronic acid byStreptococcus zooepidemicus (in Japanese). J Soc Cosmet Chem Japan 1988;22:35–42.
Amiel D, Frank C, Harwood F, et al. Tendons and ligaments: A morphological and biochemical comparison. J Orthop Res 1984; 1:257–65.
Brown AF. Neutrophil granulocytes: Adhesion and locomotion on collagen substrata and in collagen matrices. J Cell Sci 1982; 58:455–67.
Clayton ML, Miles JS, Abdulla M. Experimental investigations of ligamentous healing. Clin Orthop 1968;61:146–53.
Cortivo R, De Galateo A, Castellani I, et al. Hyaluronic acid promotes chick embryo fibroblast and chondroblast expression. Cell Biol Int 1990;14:111–22.
Feinberg RN, Beebe DL. Hyaluronate in vasculogenesis. Science 1983;220:1177–9.
Hascall VC, Heingård D. Aggregation of cartilage proteoglycans. I. The role of hyaluronic acid. J Biol Chem 1974;249:4232–41.
Hellström S, Laurent C. Hyaluronan and healing of tympanic membrane perforations. An experimental study. Acta Otolaryngol Suppl (Stockh) 1987;442:54–61.
Iwata H. Pharmacologic and clinical aspects of intraarticular injection of hyaluronate. Clin Orthop 1993;289:285–91.
Kikuchi T, Denda S, Yamaguchi T. Effect of sodium hyaluronate (SL-1010) on glycosaminoglycan synthesis and release in rabbit articular cartilage (in Japanese). Jpn Pharmacol Ther (Suppl) 1993;21:157–63.
Kimata K, Oike Y, Tani K, et al. A large chondroitin sulfate proteoglycan (PG-M) synthesized before chondrogenesis in the limb bud of chick embryo. J Biol Chem 1986;261:13517–25.
Kleiner JB, Roux RD, Amiel D, et al. Primary healing of the anterior cruciate ligament (ACL). Trans Orthop Res Soc 1986; 11:131.
LeBaron RG, Zimmermann DR, Rouslahti E. Hyaluronate binding properties of versican. J Biol Chem 1992;267:10003–10.
Marshall JL, Olssen S-E. Instability of the knee: A long-term experimental study in dogs. J Bone Joint Surg Am 1971;53:1561–70.
O'Donoghue DH, Frank GR, Jeter GL, et al. Repair and reconstruction of the anterior cruciate ligament in dogs. J Bone Joint Surg Am 1971;53:710–8.
Rydell N, Balazs EA. Effect of intra-articular injection of hyaluronic acid on the clinical symptoms of osteoarthritis and on granulation tissue formation. Clin Orthop 1971;80:25–32.
Sakakibara Y, Miura T, Iwata H, et al. Effect of high-molecular weight sodium hyaluronate on immobilized rabbit knee. Clin Orthop 1994;299:282–92.
Scott PG, Dodd CM, Tredget EE, et al. Immunohistochemical localization of the proteoglycans decorin, biglycan and versican and transforming growth factor-β in human post-burn hypertrophic and mature scars. Histopathology 1995;26:423–31.
Sherman MF, Warren RF, Marshall JL, et al. A clinical and radiographical analysis of 127 anterior cruciate insufficient knees. Clin Orthop 1988;227:229–37.
Suzuki Y, Yamaguchi T. Effects of hyaluronic acid on macrophage phagocytosis and active oxygen release. Agents Actions 1993;38:32–7.
Tsuboi I, Kotera N, Shichijo S, et al. Effect of high molecular weight sodium hyaluronate (SL-1010) on human neutrophil function. Jpn J Inflam 1993;13:55–61.
Vogel KG, Ördög A, Prgány G, et al. Proteoglycans in the compressed region of human tibialis posterior tendon and in ligaments. J Orthop Res 1993;11:68–77.
Wiig ME, Ameil D, VandeBerg J, et al. The early effect of high molecular weight hyaluronan (hyaluronic acid) on anterior cruciate ligament healing: An experimental study in rabbits. J Orthop Res 1990;8:425–34.
Weigel PH, Frost SJ, McGary CT, et al. The role of hyaluronic acid in inflammation and wound healing. Int J Tiss Reac 1988; 10:355–65.
West DC, Kumar S. The effect of hyaluronate and its oligosaccharides on endothelial cell proliferation and monolayer integrity. Exp Cell Res 1989;183:179–96.
Yamagata M, Yamada KM, Yoneda M, et al. Chondroitin sulphate proteoglycan (PG-M-like proteoglycan) is involved in the binding of hyaluronic acid to cellular fibronectin. J Biol Chem 1986;261:13526–35.
Yoshimi T, Kikuchi T, Obara T, et al. Effects of high-molecular-weight sodium hyaluronate on experimental osteoarthrosis induced by the resection of rabbit anterior cruciate ligament. Clin Orthop 1994;298:296–304.
Zimmermann DR, Dours-Zimmermann MT, Schubert M, et al. Versican is expressed in the proliferating zone in the epidermis and in association with the elastic network of the dermis. J Cell Biol 1994;124:817–25.
Author information
Authors and Affiliations
About this article
Cite this article
Kikuchi, T., Fukui, N., Sakuta, T. et al. Effect of hyaluronan on the healing of partially ruptured anterior cruciate ligament of rabbits. J Orthop Sci 2, 31–39 (1997). https://doi.org/10.1007/BF01239756
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01239756