Summary
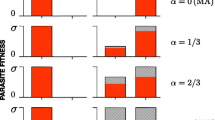
A model of host—parasite coevolution is analysed. A host resistance trait and a parasite virulence trait interact to determine the outcome of a parasitic attack, where each trait is determined by quantitative genetic variation. The resistance and virulence traits are assumed to have a fitness cost. Each host and parasite genotype is treated as a separate ‘species’ in a multidimensional Lotka—Volterra system in which the numerical abundance of each genotype is free to change. Thus, the epidemiological effects of fluctuating population sizes are analysed jointly with changes in genotype frequencies. Population sizes fluctuate increasingly as the parasites' reproductive capacity increases and as resistance and virulence benefits per unit cost decline. The patterns of genetic variability depend mainly on the stability of population sizes and on the shape of the relationship between the costs and benefits of a trait.
Similar content being viewed by others
References
Alexander, H.M. (1989) An experimental field study of anther-smut disease ofSilene alba caused byUstilago violacea: genotypic variation and disease resistance.Evolution 43, 835–47.
Alexander, H.M. (1990) Dynamics of plant—pathogen interactions in natural plant communities. InPests, Pathogens and Plant Communities (J.J. Burdon and S.R. Leather, eds), pp. 31–45. Blackwell Scientific, Oxford, UK.
Alexander, H.M. and Antonovics J. (1988) Disease spread and population dynamics of anther-smut infection ofSilene alba caused by the fungusUstilago violacea.J. Ecol. 76, 91–104.
Berenbaum, M.R. and Zangerl, A.R. (1992) Quantification of chemical coevolution. InPlant Resistance to Herbivores and Pathogens (R.S. Fritz and E.L. Simms, eds), pp. 69–87. University of Chicago Press, Chicago, USA.
Berenbaum, M.R., Zangerl, A.R. and Nitao, J.K. (1986) Constraints on chemical coevolution: wild parsnips and the parsnip webworm.Evolution 40, 1215–28.
Burdon, J.J. (1987)Diseases and Plant Population Biology. Cambridge University Press, Cambridge, UK.
Christ, B.J., Person, C.O. and Pope, D.D. (1987) The genetic determination of variation in pathogenicity. InPopulations of Plant Pathogens (M.S. Wolfe and C.E. Caten, eds), pp. 7–19. Blackwell Scientific, Oxford, UK.
Frank, S.A. (1990) Sex allocation theory for birds and mammals.Ann. Rev. Ecol. System. 21, 13–55.
Frank, S.A. (1991a) Spatial variation in coevolutionary dynamics.Evol. Ecol. 5, 193–217.
Frank, S.A. (1991b) Ecological and genetic models of host—pathogen coevolution.Heredity 67, 73–83.
Frank, S.A. (1992) Models of plant—pathogen coevolution.Trends Genet. 8, 213–19.
Frank, S.A. (1993) Coevolutionary genetics of plants and pathogens.Evol. Ecol. 7, 45–75.
Frank, S.A. and Slatkin, M. (1990) The distribution of allelic effects under mutation and selection.Genet. Res. 55, 111–17.
Fry, J.D. (1992) On the maintenance of genetic variation by disruptive selection among hosts in a phytophagous mite.Evolution,46, 279–83.
Gould, F. (1983) Genetics of plant—herbivore systems: interactions between applied and basic studies. InVariable Plants and Herbivores in Natural and Managed Systems (R.F. Denno and M.S. McClure, eds), pp. 599–653. Academic Press, New York, USA.
Guckenheimer, J. and Holmes, P. (1983)Nonlinear Oscillations, Dynamical Systems, and Bifurcations of Vector Fields. Springer-Verlag, New York, USA.
Hamilton, W.D. (1986) Instability and cycling of two competing hosts with two parasites. InEvolutionary Processes and Theory (S. Karlin and E. Nevo, eds), pp. 645–68. Academic Press, New York, USA.
Kennedy, G.G. and Barbour, J.D. (1992) Resistance variation in natural and managed systems. InPlant Resistance to Herbivores and Pathogens (R.S. Fritz and E.L. Simms, eds), pp. 13–41. University of Chicago Press, Chicago, USA.
Leonard, K.J. and Czochor, R.J. (1980) Theory of genetic interactions among populations of plants and their pathogens.Ann. Rev. Phytopathol. 18, 237–58.
Marquis, R.J. and Alexander, H.M. (1992) Evolution of resistance and virulence in plant—herbivore and plant—pathogen interactions.Trends Ecol. Evol. 7, 126–9.
May, R.M. (1974)Stability and Complexity in Model Ecosystems, 2nd edn. Princeton University Press, Princeton, NJ, USA.
Mitter, C. and Futuyma, D.J. (1983) An evolutionary—genetic view of host-plant utilization by insects. InVariable Plants and Herbivores in Natural and Managed Systems (R.F. Denno and M.S. McClure, eds), pp. 427–59. Academic Press, New York, USA.
Rausher, M.D. and Simms, E.L. (1989) The evolution of resistance to herbivory inIpomoea purpurea. I. Attempts to detect selection.Evolution 43, 563–72.
Saloniemi, I. (1993) A coevolutionary predator—prey model with quantitative characters.Am. Nat. 141, 880–96.
Seger, J. (1992) Evolution of exploiter—victim relationships. InNatural Enemies: the Population Biology of Predators, Parasites and Diseases (M.J. Crawley, ed) pp. 3–25. Blackwell Scientific, Oxford, UK.
Simms, E.L. and Fritz, R.S. (1990) The ecology and evolution of host-plant resistance to insects.Trends Ecol. Evol. 5, 356–60.
Simms, E.L. and Rausher, M.D. (1987) Costs and benefits of plant resistance to herbivory.Am. Nat. 130, 570–81.
Simms, E.L. and Rausher, M.D. (1989) The evolution of resistance to herbivory inIpomoea purpurea. II. Natural selection by insects and costs of resistance.Evolution 43, 573–85.
Slatkin, M. (1987) Heritable variation and heterozygosity under a balance between mutation and stabilizing selection.Genet. Res. 50, 53–62.
Via, S. (1991) The genetic structure of host plant adaptation in a spatial patchwork: demographic variability among reciprocally transplanted pea aphid clones.Evolution 45, 827–52.
Wolfram, S. (1991)Mathematica, 2nd edn. Addison-Wesley, Redwood City, CA, USA.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Frank, S.A. Coevolutionary genetics of hosts and parasites with quantitative inheritance. Evol Ecol 8, 74–94 (1994). https://doi.org/10.1007/BF01237668
Issue Date:
DOI: https://doi.org/10.1007/BF01237668