Abstract
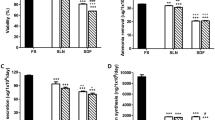
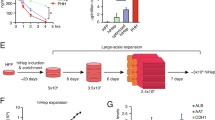
Glutamine synthetase-transduced Chinese hamster ovary (GS-CHO) cells were cultured in a circulatory flow bioreactor for 28 days. The bioreactor inoculated with 4.72×108 of GS-CHO cells was kept at 37°C and supplied with air, CO2, O2, and glutamine-free RDF culture medium containing 5% fetal bovine serum, 2.3mM of ammonia, and 1.0mM of glutamate. Since GS-CHO cells synthesize glytamine from ammonia and glutamate, the ammonia concentration in the culture medium was decreased corresponding to the cell growth. The clearance rate of added ammonia showed that the ammonia removal activity was equivalent to that of 1.25×108 porcine hepatocytes. Amino acid analysis showed a peak of glutamine synthesis that was not found in the original medium. The ammonia concentration in the culture medium returned to the original level 24 days after the start of culture, suggesting the cells went into a stationary stage. The number of cells in the bioreactor at the end of the culture was 9.64×109, and the cells were uniformly distributed throughout the glass fiber cell matrix. These data suggest that the circulatory flow bioreactor system with recombinant cells is applicable for a bioartificial liver assist unit to remove toxic substances in the blood of a patient whose detoxification has been injured by hepatic failure.
Similar content being viewed by others
References
Williams R. Classification, etiology, and considerations of outcome in acute liver failure. Semin Liver Dis 1996;16:343–348
Yoshiba M, Inoue K, Sekiyama K, Koh I. Favorable effect of new artificial liver support on survival of patients with fulminant hepatic failure. Artif Organs 1996;20:1169–1172
Matsubara S, Okabe K, Ouchi K, Sato T, Matsuno S. Temporary metabolic support by extracorporeal blood therapy for liver failure after surgery. ASAIO Trans 1988;34:266–269
Takahashi Y, Shimizu M. Etiology and prognosis of fulminant viral hepatitis in Japan: a multicentre study. The Study Group of Fulminant Hepatitis. J Gastroenterol Hepatol 1991;6:159–164
MacLaughlin BE, Tosone CM, Custer LM, Mullon C. Overview of extracorporeal liver support systems and clinical results Ann NY Acad Sci 1999;875:310–325
Cousineau J, Chang TM. Formation of amino acid from urea and ammonia by sequential enzyme reaction using a microencapsulated multi-enzyme system. Biochem Biophys Res Commun 1977;79:24–31
Enosawa S, Suzuki S, Kakefuda T, Amemiya H, Omasa T, Suga K, Ito N, Kuramochi K. Estimation of ammonia removal activity of glutamine synthetase immobilized gel with the aim of selective detoxification of blood ammonia (abstract in English). Jpn J Artif Organs 1996;25:399–402
Enosawa S, Suzuki S, Fujino M, Amemiya H, Omasa T, Urayama S, Tanimura N, Suga K. An attempt to add biological functions by genetic engineering in order to produce high-performance bioreactor cells for hybrid artificial liver: transfection of glutamine synthetase into Chinese hamster ovary (CHO) cell. Cell Transplant 1997;6:537–540
Omasa T, Urayama S, Yamanaka M, Tanimura N, Katakura Y, Kishimoto M, Suga K, Enosawa S, Amemiya H. Construction of ammonia metabolizing cell line for artificial hybrid liver support system using recombinant DNA technique (abstract in English). Kagaku Kogaku Ronbunshu 1998;24:184–189
Wilson RH. GS gene amplification in CHO cells. In: Kelems RE (ed) Gene amplification in mammalian cells: a comprehensive guide. New York: Marcel Dekker, 1993, pp 301–311
Matsumura T, Sawai Y, Suzuki J, Fujimori T. Circulatory culture equipment. 1993; U.S. Patent 5270207
Ijima H, Nakazawa K, Kaneko M, Mizumoto H, Matsushita T, Gion T, Shimada M, Shirabe K, Takenaka K, Sugimachi K, Funatsu K. The formation of a spheroid of primary hepatocytes and the expression of liver-specific functions depend on the characteristics of polyurethane foam. J Artif Organs 1998;1:83–88
Detry O, Arkadopoulos N, Ting P, Kahaku E, Watanabe FD, Rozga J, Demetriou AA. Clinical use of a bioartificial liver in the treatment of acetaminophen-induced fulminant hepatic failure. Am Surg 1999;65(10):934–938
Busse B, Gerlach JC. Bioreactors for hybrid liver support: historical aspects and novel designs. Ann NY Acad Sci 1999;875:326–339
Michaels MG. Xenotransplant-associated infections. Lab Anim Sci 1998;48:228–233
Patience C, Takeuchi Y, Weiss R. Infection of human cells by an endogenous retrovirus of pigs. Nature Med 1997;3:282–286
Enosawa S, Suzuki S, Kakefuda T, Amemiya H. Examination of 7-ethoxycoumarin deethylation and ammonia removal activities in hepatocytes cell lines. Cell Transpl 1996;5:S39–40
Yanagi K, Miyoshi H, Ohshima N. A high density culture of hepatocytes using porous substrate for use as a bioartificial liver. J Artif Organs 1999;2:124–128
Kong D, Cardak S, Chen M, Gentz R, Zhang J. High density and productivity culture of Chinese hamster ovary cells in a fluidized bed bioreactor. Cytotechnology 1999;29:215–220
Chiou T-W, Murakami S, Wang DIC, Wu W-T. A fiber-bed bioreactor for anchorage-dependent animal cell cultures: Part I. bioreactor design and operations. Biotech Bioeng 1991;37:755–761
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Enosawa, S., Mukaiyama, T., Miyashita, T. et al. Application of circulatory flow bioreactor for long-term and large-scale culture of glutamine synthetase transduced CHO cells and its ammonia removal activity with an aim of development for a bioartificial liver assist system. J Artif Organs 4, 61–66 (2001). https://doi.org/10.1007/BF01235838
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01235838