Abstract
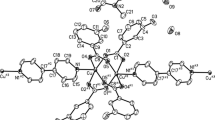
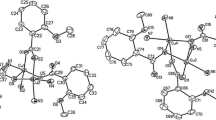
The dimeric Cu(I) complex [(μ-dppf)(Cu(dppf))2](ClO4)2, where dppf is Fe((n5-C5H4)P-(C6H5)2)2, has been characterized by single-crystal X-ray crystallography. The complex crystallizes in the triclinic space groupP1 (no. 2) with unit cell parametersa=21.852(5),b=18.063(5),c=14.867(5)Å, α=114.32(3), β=109.87(3), γ=82.84(3)°. The structure was refined toR=5.40% based on 5562 observed reflections. Two crystallographically independent, structurally equivalent, units were observed in the structure. The structure consists of the centrosymmetric dimeric [(μ-dppf) (Cu(dppf))2]2+ cations and ClO4/− anions. The copper atoms are three-coordinate being trigonally bonded to one chelating and one bridging ligand. The P-Cu-P bond angles for the chelating dppf ligand are 111.1(2)° and 111.3(2)°, while the P-Cu-P angles linking different dppf molecules range from 123.9(2) to 124.2(2)°. Cu-P bond distances fall within normal ranges lying between 2.265(5) and 2.285(4)Å, with the values for the chelating dppf being slightly larger than those observed for the bridging ligand. The cyclopentadienyl rings are exactly staggered and parallel, and the P atoms 180° opposed in the bridging dppf, while the rings maintain an approximately staggered configuration and are inclined by 1.6° to each other with the P atoms being 39.5° apart in the chelating ligand. A steric control of the stereochemistry of the monomer fragment of the dimer complex is suggested.
Similar content being viewed by others
References
Baiada, A., Jardine, F. H., Willet, R. D., and Emerson, K. (1991)Inorg. Chem. 30, 1365.
Butler, I. R., Cullen, W. R., Kim, T., Rettig, S. J., and Trotter, J. (1985)Organometallics 4, 972.
Camus, A., Marsich, N., Nardin, G., and Randaccio, L. (1976)Trans. Met. Chem. 1, 205.
Casellato, U., Ajó, D., Valle, G., Corain, B., Longato, B., and Graziani, R. (1988)J. Cryst. Spec. Res. 18, 583.
Casellato, U., Corain, B., Graziani, R., Longato, B., and Pilloni, G. (1990)Inorg. Chem. 29, 1193.
Clemente, D. A., Pilloni, G., Corain, B., Longato, B., and Tiripicchio-Camellini, M. (1986)Inorg. Chim. Acta 115, L9.
Corain, B., Longato, B., Favero, G., Ajó, D., Pilloni, G., Russo, U., and Kreissel, F. R. (1989)Inorg. Chim. Acta 157, 259.
Cromer, D. T., and Libermann, D. (1970)J. Chem. Phys. 53, 1891.
Cullen, W. R., Kim, T., Einstein, F. W. B., and Jones, T. (1985)Organometallics 4, 346.
Darensbourg, D., Chao, C.-S., Reibenspies, J., and Bischoff, C. J. (1990)Inorg. Chem. 29, 2153.
Hayashi, T., Konishi, M., Kobari, Y., Kumada, M., Higuchi, T., and Hirotsu, K. (1984)J. Am. Chem. Soc. 106, 158.
Hill, D. T., Girard, G. R., McCabe, F. L., Johnson, R. K., Stupik, P. D., Zhang, J. H., Reiff, W. M., and Eggleston, D. S. (1989)Inorg. Chem. 28, 3529.
Leoni, P., Pasquali, M., and Ghilardi, C. A. (1983)J. Chem. Soc. Chem. Commun. 240.
Longato, B., Pilloni, G., Valle, G., and Corain, B. (1988)Inorg. Chem. 27, 956.
Longato, B., Pilloni, G., Graziani, R., and Casellato, U. (1991)J. Organomet. Chem. 407, 369.
Miller, T, M., Kazi, J. A., and Wrighton, M. S. (1989)Inorg. Chem. 28, 2347.
Mohr, B., Brooks, E. E., Rath, N., and Deutsch, E. (1991)Inorg. Chem. 30, 4541.
North, A. C. T., Phillips, D. C., and Mathews, F. S. (1968)Acta Cristallogr. A 24, 351.
Onaka, S. (1986)Bull. Chem. Soc. Jpn. 59, 2359.
Pilloni, G., Longato, B., and Corain, B. (1991)J. Organomet. Chem. 420, 57.
Pilloni, G., Graziani, R., Longato, B., and Corain, B. (1991)Inorg. Chim. Acta 190, 165.
Sheldrick, G. M. (1976)Shelx Program for crystal structure determination (Univ. of Cambridge).
Stephan, D. W. (1989)Coord. Chem. Rev. 95, 41.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Casellato, U., Graziani, R. & Pilloni, G. Heteropolymetallic complexes of 1,1′-bis (diphenylphosphino)ferrocene, dppf. VIII. X-ray crystal structure of copper(I) dimer [(μ-dppf)(Cu(dppf))2](ClO4)2 1 . Journal of Crystallographic and Spectroscopic Research 23, 571–575 (1993). https://doi.org/10.1007/BF01228767
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01228767