Objective
The purpose of this study was to elucidate changes in the distribution of mitochondria through the cell cycle.
Materials and Methods
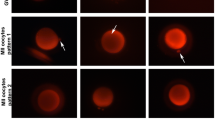
Mouse oocytes and embryos were recovered sequentially from mice and stained with the vital fluorescent mitochondrial stain rhodamine 123. Mitochondrial staining pattern were classified into three types: aggregation (Ag), homogeneous (H), and perinuclear accumulation (PA).
Results
Sequential observations revealed that mitochondria of oocytes and embryos grown in vivo translocated in the cytoplasm during the cell cycle, showing the H pattern be fore human chorionic gonadotropin (hCG) administration, the PA pattern 8–9 hr post-hCG, the H pattern again 10–14 hr post-hCG, and the PA pattern again 24 and 31–32 hr post-hCG following fertilization. In the twocell stage, the Ag pattern was shown 35 hr post-hCG, the H pattern was observed 40 hr post-hCG, and the PA pattern was found 48 hr post-hCG. In the embryos cultured in vitro and showing developmental block, mitochondrial translocation was shown to be inhibited after they aggregated in the early two-cell stage (35 hr post-hCG). Moreover, the translocation of mitochondria was restored by the addition of superoxide dismutase or thioredoxin to the culture medium. Both of these enzymes have already been shown to have the ability to overcome developmental block.
Conclusion
The present study revealed that mitochondria translocated in the cell cycle and suggested that there is a close relationship between mitochondrial translocation and developmental arrest.
Similar content being viewed by others
References
Lehninger AL: Molecular basis of structure and function.In The Mitochondrion, WA Benjamin (ed). Menlo Park, CA, 1964, pp 1–70
Palade GE: Units of biological structure and function.In Enzymes, OH Gaebler (ed.). New York, Academic Press, 1956, pp 185–215
De Robertis EDP, Nowinski WW, Saez FA: Cell Biology. New York, WB Saunders, 1965
Rouiller C: Physiological and pathological changes in mitochondrial morphology. Int Rev Cytol 1960;9:227–292
Packer L: Size and shape transformations correlated with oxidative phosphorylation in mitochondria. I. Swelling-shrinkage mechanisms in intact mitochondria. J Cell Biol 1963;18:487–494
Hackenbrock CR: Ultrastructural bases for metabolically linked mechanical activity in mitochondria. I. Reversible ultrastructural changes with change in metabolic steady state in isolated liver mitochondria. J Cell Biol 1966;30:269–297
Hackenbrock CR: Ultrastructural bases for metabolically linked mechanical activity in mitochondria. II. Electron transport-linked ultrastructural transformations in mitochondria. J Cell Biol 1968;37:345–369
Rosenbaum RM, Wittner M, Lenger M: Mitochondrial and other ultrastructural changes in great alveolar cells of oxygen-adapted and poisoned rats. Lab Invest 1969;20:516–528
Weakley BS: Variations in mitochondrial size and ultrastructure during germ cell development. Cell Tissue Res 1976;169:531–550
Heggeness MH, Simon M, Singer SJ: Association of mitochondria with microtubules in cultured cells. Proc Natl Acad Sci USA 1978;75(8):3863–3866
Johnson LV, Walsh ML, Chen LB: Localization of mitochondria in living cells with rhodamine 123. Proc Natl Acad Sci USA 1980;77(2):990–994
Van Blerkom J, Runner MN: Mitochondrial reorganization during resumption of arrested meiosis in the mouse oocyte. Am J Anat 1984;171:335–355
Muggleton-Harris AL, Brown JJG: Cytoplasmic factors influence mitochondrial reorganization and resumption of cleavage during culture of early mouse embryos. Hum Reprod 1988;3(8):1020–1028
Yanagimachi R, Chang MC: Fertilizable life of golden hamster ova and their morphological changes at the time of losing fertilizability. J Exp Zool 1961;148:1–14
Biggers JD, Whitten WK, Whittingham DG: The culture of mouse embryos in vitro.In Methods in Mammalian Embryology, Daniel JC (ed.). San Francisco, WH Freeman, 1971, pp 86–116
Pratt HPM, Muggleton-Harris AL: Cycling cytoplasmic factors that promote mitosis in the cultured 2-cell mouse embryo. Development 1988;104:115–120
Stern S, Biggers JD, Anderson E: Mitochondria and early development of the mouse. J Exp Zool 1971;176:179–192
Piko L, Taylor KD: Amounts of mitochondrial DNA and abundance of some mitochondrial gene transcripts in early mouse embryos. Dev Biol 1987;123:364–374
Rancourt MW, McCarthy AP, Pollack WJ: Mitochondrial profile of a mammalian lymphocyte. J Ultrastruct Res 1975;51:418–424
Koukl JF, Vorbeck ML, Martin AP: Mitochondrial three-dimensional form in ascites tumor cells during changes in respiration. J Ultrastruct Res 1977;61:158–165
Bakeeva LE, Chentsov YS, Skulachev VP: Mitochondrial framework (reticulum mitochondriale) in rat diaphragm muscle. Biochim Biophys Acta 1978;501:349–369
Wang E, Goldman RD: Functions of cytoplasmic fibers in intracellular movements in BHK-21 cells. J Cell Biol 1978;79:708–726
Summerhayes IC, Wong D, Chen LB: Effect of microtubules and intermediate filaments on mitochodrial distribution. J Cell Sci 1983;61:87–105
Ball EH, Singer SJ: Mitochondria are associated with microtubulesand not with intermediate filaments in cultured fibroblasts. Proc Natl Acad Sci USA 1982;79:123–126
Schliwa M: Mechanisms of intracellular organelle transport.In Cell and Muscle Motility, Shay JW (ed). New York, Plenum, 1984, pp 1–82
Schatten G, Simerly C, Schatten H: Microtubule configurations during fertilization, mitosis, and early development in the mouse and the requirement for egg microtubulemediated motility during mammalian fertilization. Proc Natl Acad Sci USA 1985;82:4152–4156
Van Blerkom J: Microtubule mediation of cytoplasmic and nuclear maturation during the early stages of resumed meiosis in cultured mouse oocytes. Proc Natl Acad Sci USA 1991;88:5031–5035
Van Cauwenberge A, Alexandre H: Control of microtubule nucleating activity in the cytoplasm of maturing mouse oocytes. Int J Dev Biol 1992;36:143–150
Wright RJ, Bondioli KR: Aspects of in vitro fertilization and embryo culture in domestic animals. J Anim Sci 1981;53:702–728
Fisher B: Developmental retardation in cultured preimplantation rabbit embryos. J Reprod Fertil 1987;79:115–123
Whitten WK: Culture of tubal ova. Nature 1957;179:1081–1082
Yanagimachi R, Chang MC: In vitro fertilization of golden hamster ova. J Exp Zool 1964;156:361–376
Whittingham DG: Fertilization, early development and storage of mammalian ova in vitro.In The Early Development of Mammals; Br Soc Dev Biol Symp 2, M Balis, AE Wild (ed). Cambridge, Cambridge University Press, 1975, pp 1–24
Sakai H, Ohta K: Centrosome signalling at mitosis. Cell Signal 1991;3:267–272
Natsuyama S, Noda Y, Nagahama Y, Mori T: Superoxide dismutase and thioredoxin restore defective p34cdc2 kinase activation in mouse two-cell block. Biochim Biophys Acta 1993;1176(1–2):90–94
Noda Y, Matsumoto H, Umaoka Y, Tatsumi K, Kishi J, Mori T: Involvement of superoxide radicals in the mouse 2-cell block. Mol Reprod Dev 1991;28:356–360
Umaoka Y, Noda Y, Narimoto K, Mori T: Effects of oxygen toxicity on early development of mouse embryos. Mol Reprod Dev 1992;31:28–33
Goto Y, Noda Y, Narimoto K, Umaoka Y, Mori T: Oxidative stress on embryo development in vitro. Free Radical Biol Med 1992;13:47–53
Natsuyama S, Noda Y, Narimoto K, Umaoka Y, Mori T: Release of mouse two-cell block by reduction of protein disulphide with thioreoxin from Escherichia coli. J Reprod Fertil 1992;95:649–656
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tokura, T., Noda, Y., Goto, Y. et al. Sequential observation of mitochondrial distribution in mouse oocytes and embryos. J Assist Reprod Genet 10, 417–426 (1993). https://doi.org/10.1007/BF01228092
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01228092